Non-covalent bonds between electron donors and silicon, tin or germanium are strong but overlooked
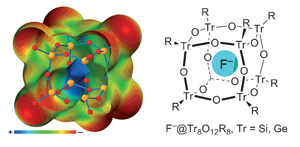
Researchers have coined the term ‘tetrel bonding’ to highlight little-studied but powerful non-covalent bonding between electron donors and the group 14 elements, silicon, germanium and tin.1 Calculations by Antonio Frontera of the University of the Balearic Islands and his teammates in Spain and the UK also explore how these interactions are influencing modern chemistry. ‘Tetrel bonds have at least comparable strength to hydrogen bonds and other s-hole interactions,’ Frontera tells Chemistry World.
A ‘s-hole’ is a low electron density, and therefore relatively positively charged, area adjacent to a s-bonded element from groups 14 to 17. These areas appear on the opposite side of such atoms to the bond, along the same axis. The positive charges let the atoms act as Lewis acids, and bond non-covalently with electron donors. Halogen bonding is perhaps the best known such interaction, involving group 17 elements. And in recent years s-hole bonding involving group 16 and 15 elements have named according to the groups’ trivial names, as chalcogen and pnicogen bonding respectively.
Scientists had already spotted silicon acting as a Lewis acid and form dative bonds with nitrogen and halogens around the turn of the millennium. However, few had done detailed modern quantum chemistry calculations on how this exploits group 14 bonds’ s-hole charges. ‘Consequently, we extended this type of non-covalent interaction to these “tetrel” elements,’ Frontera explains.
Among other findings, Frontera’s team’s calculations suggest that tetrel bonding helps tetra-n-butylammonium fluoride catalyse spherosilicate synthesis, via fluoride ions trapped inside siloxane cubes.2 Their values for moderately strong non-covalent interactions, at 11kcal/mol between fluoride and silicon, agree with NMR data. Calculations replacing silicon with germanium and tin also raise bond strengths to 14 and 15 kcal/mol respectively, increasing in the heavier atoms as other s-hole interactions do. This shows how powerful these bonds can be, Frontera notes. ‘I expect tetrel bonding interactions will be valuable for crystal engineering, supramolecular chemistry, and catalysis,’ he adds.
Jane Murray, a computational chemist at the University of New Orleans, agrees broader understanding of these interactions could help in supramolecular chemistry. But she is resistant to calling them ‘tetrel bonding’, having recently called for all interactions involving s-holes to be united under one title.3 ‘Group 14 interactions have been noted as s-hole interactions since 2009,’ she underlines. But Frontera emphasises that an easily recognisable name could help raise the interactions’ profile. ‘The tetrel bonding interaction has been overlooked in several fields,’ he stresses.







No comments yet