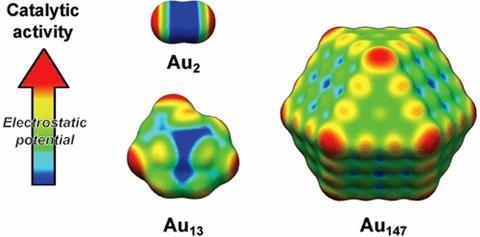
Calculations show low electron density makes nanoparticle corners and edges reactive
Researchers have devised what may be the first physical explanation for a key feature of how gold nanoparticle catalysts work. Scientists already knew gold nanoparticles’ unique catalytic properties come because they bind Lewis base reactant molecules, which feature an electron pair, at their edges and corners. But now calculations by Joakim Halldin Stenlid and Tore Brinck at KTH Royal Institute of Technology in Stockholm, Sweden, indicate how the metal’s electrons drive this effect.
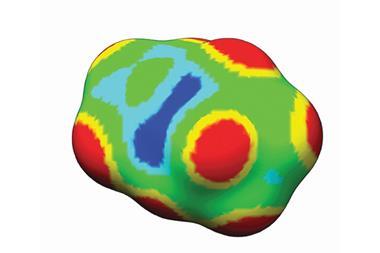
Specifically, they find edge and corner areas have low electron density, which can also be considered as positive electrostatic potential. ‘The most catalytically active sites of gold particles are associated with positive surface electrostatic potential, and magnitude of the electrostatic potential correlates with the activity,’ Brinck tells Chemistry World.
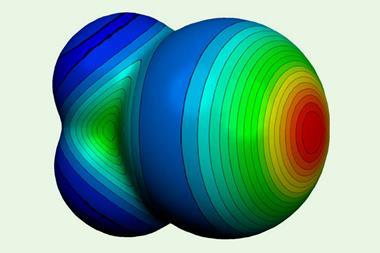
Brinck and Stenlid emphasise that positive potential areas are ‘σ-holes’, meaning that they can generate an intermolecular attraction to electron-rich atoms, such as those in Lewis bases. This effect, known as σ-hole bonding, is also seen with low electron density σ-hole areas on halogen atoms, for example, and even hydrogen bonding. It comes partly because gold is similar to hydrogen in having a lone s-orbital electron.
The way catalysis scientists normally use density functional theory (DFT) calculations makes it hard to study surface electrostatic potentials precisely, Brinck says. But because the KTH researchers come from a quantum chemistry background, they realised that they didn’t need such great precision. Instead, they could investigate general potential patterns relatively easily, even for large systems.
First they simulated diatomic gold molecules, finding that, like diatomic halogen molecules, positive electrostatic potentials formed on the end of the atoms opposite the bond between them. Building up clusters containing more gold atoms, the simulations showed positive potential areas concentrated on atoms with the fewest neighbours – namely those at edges and corners.
‘The extension of the σ-hole concept to noble metals like gold is really novel and appealing,’ comments σ-hole expert Antonio Frontera from the University of the Balearic Islands in Spain. ‘I am convinced that this will pave the way to a new field of research.’
The KTH scientists now want to analyse other metal and metal oxide catalysts, particularly the importance of shape and chemical composition on activity, and identify active sites of more complex catalysts. They also hope the method could yield a working tool for catalyst design, Brinck says.
References
J H Stenlid and T Brinck, J. Am. Chem. Soc., 2017, DOI: 10.1021/jacs.7b05987












No comments yet