Coupling unactivated phenols with amines requires an unusual approach, as Karl Collins discovers
Catalytic dehydrogenative cross-coupling reactions – in which two C–H bonds are activated to form a C–C bond – are, in many ways, considered the ideal cross-coupling. They require no pre-functionalisation of reactants, and produce only hydrogen as a stoichiometric by-product (figure 1).
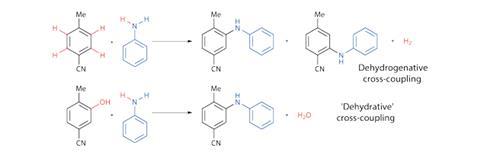
The analogous ‘dehydrative’ reactions, coupling an X–H bond and a phenol, are arguably preferable in terms of by-products – liberating water instead of flammable hydrogen gas. And since polymeric phenols derived from renewable lignin biomass are an increasingly viable source of chemical building blocks, these reactions are more attractive from the standpoint of sustainability. Furthermore, the hydroxyl group dictates the site of reaction – removing selectivity issues – and it can also be used as a synthetic handle in preceding reactions.
Despite the attractiveness of unactivated phenols in coupling reactions, the very strong C–O bond has provided a major hurdle to their use. Activated phenol derivatives, such as aromatic triflates, are more widely employed owing to easier oxidative addition at metal centres. More recently, reactions of arylcarbonates, ethers and phenolic salts have been developed. In contrast, the parent phenols are underexploited, and a different strategic approach was required from Chao-Jun Li and colleagues at McGill University, Canada, to enable a palladium-catalysed cross-coupling of phenols with amines.
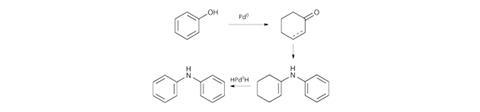
Instead of employing the oxidative addition and reductive elimination processes of the widely used Buchwald–Hartwig C–N coupling reaction, Li and his team exploit a transfer hydrogenation mechanism. The team proposed that by reducing the phenol to a cyclic ketone, condensation could occur with an amine, and the formally cross-coupled product could be isolated after re-oxidising the reduced ring (figure 2).
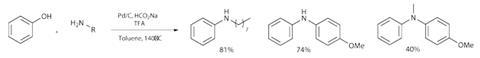
The reaction is a very simple ‘combine and stir’ procedure, run under an inert atmosphere. Initially, reacting a slight excess of octylamine with phenol in toluene with 10mol% palladium on carbon as the catalyst and sodium formate as a reductant gives the amine product in 52% isolated yield. Adding half an equivalent of trifluoroacetic acid and increasing the temperature from 120°C to 140°C bumps this significantly to 81% (figure 3). The researchers propose the acid likely helps with the condensation of the amine and ketone, but related studies have shown the condensation itself proceeds very well under near identical conditions without any additional acid.
The scope is not huge, but primary and secondary amines as well as electronically distinct anilines react efficiently (40–85% yield), and a number of simple phenols and naphthols react well. Exceptional examples include reactions with methoxy- and acetamide-substituted phenols, although aryl chlorides are reduced under the reaction conditions (as might be expected). The reaction clearly has limitations, and with reduction of the arene as an elementary step, extending its scope will definitely be a challenge. But nevertheless, direct coupling of unactivated phenols is a very attractive process.
The team has yet to look into the mechanism in any great detail, although basic experiments do support the feasibility of ketones or enones as reaction intermediates. The team didn’t discuss the nature of the active catalyst – hetero- or homogeneous. Despite Pd/C formally being a heterogeneous source of palladium, with the reaction running at 140°C, leaching of palladium from the catalyst surface is almost certain, meaning no conclusions can be drawn as to whether the reaction occurs on the catalyst surface or in solution. Some insight may be gleaned from a short screen of palladium sources during optimisation, with homogeneous palladium sources – except Pd(OAc)2 – giving traces of reaction at best. As Pd(OAc)2 has a tendency to decompose into heterogeneous palladium species at high temperatures, it could be an initial indication that the reaction may be a heterogeneous process and this is hopefully something the team will look into in the future.
The direct ‘cross-coupling’ of phenols with amines and anilines has huge potential, and this work is very exciting. But as is too often the case when new methods are developed, the absence of any information on which substrates didn’t work puts me off investing time to try it out. Another hurdle is the huge difference in reactivity of the multitude of commercial Pd/C sources available. When no information is provided, it is hard to know where to start when you have a cupboard full of catalysts. All complaining aside, the ease of running this reaction overcame any reticence on my part, and the first successful reactions in our lab are already finished!
Karl Collins leads a medicinal chemistry laboratory in Germany
References
Z Chen et al, Angew. Chem., Int. Ed., 2015, 54, 14487 (DOI: 10.1002/anie.201506751)












No comments yet