A new concept to better describe some chemical reactions
Today’s chemistry is a wide field of science with many different subfields that are all uniquely important for advancing our fundamental understanding of the properties of matter and how to manipulate it. Many of these subfields started to bloom after someone named them, thus allowing researchers to gather under a common banner. For instance, supramolecular interactions such as hydrogen bonds were studied long before Jean-Marie Lehn coined the term supramolecular chemistry in 1978,1 yet the developments in this field have increased significantly since then to become one of the leading fields of chemistry.
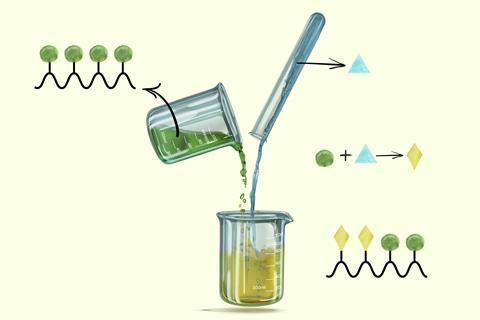
As chemists working in the field of oligomeric macrocycles, we regularly came across reactions that selectively modified the same functional group on a given number of monomers. This is a peculiar type of selectivity that is not covered by the common selectivities in organic synthesis. Indeed, these reactions modify the same type of functional group so it is not a matter of chemoselectivity. The products differing by the number of reacted groups are not isomers, thus neither regioselectivity nor stereoselectivity would apply.
The effect is not limited to oligomeric macrocycles. There has been significant effort from chemists to find selective reactions that modify a given number of functional groups on various substrates that bear two or more identical groups. These include difunctional alkanes, aromatic rings with multiple reactive C–H bonds, sugars with multiple alcohol groups or even fullerene, with a whopping 30 identical reactive sites on the Buckminsterfullerene C60. Yet, we could not find any attempt to name or formalise this type of selectivity.
We took the initiative to coin the term iteroselectivity in 2014.2 This expresses the selectivity that governs the number of repeating chemical transformations that occur on a substrate bearing multiple identical functional groups, or that occur when the reactive group is regenerated, like in some cases of polymerisation. We termed the different products from such repeating chemical transformations iteromers. We chose the prefix itero- because this selectivity happens in one-pot chemical processes where each group reacts iteratively.
On several occasions, mainly during symposia, we met fellow chemists who were working on iteroselective reactions and were looking for a term to describe this selectivity. They had never heard of iteroselectivity but were happy to learn that we proposed a word that would meet their needs. These interactions made us realise that our proposed terminology would hardly emerge naturally out of our field of oligomeric macrocycles.
With that in mind and to illustrate the applicability of the concept of iteroselectivity to a wider scope than our own oligomeric macrocycle field, we published a perspective with an extended definition of the related terminology and various examples of reactions reported in the literature that fit the definition of an iteroselective reaction, such as the mono-functionalisation of polyethylene glycols, the tetra-functionalisation of fullerene C60 via supramolecular protection, or the size-selective templated synthesis of cyclooligomers.3
Reported examples of supramolecular protection usually display impressively high iteroselectivity
We are particularly interested in examples using supramolecular protection as we are actively working on related projects. This method exploits the ability of a molecular receptor (a host) to change the local environment of a guest species in a host–guest complex. When the guest is a polyfunctional substrate that can undergo iterative reactions, the host can isolate one or several of its reactive sites from the reaction medium and reagents while leaving other reactive sites free to react. Unlike classical covalent protecting groups that require a deprotection step (sometimes in harsh conditions), the final guest product can be simply released from the host–guest complex. Reported examples of supramolecular protection usually display impressively high iteroselectivity.4
Now, achieving a seemingly iteroselective reaction raises the questions ‘what is the degree of iteroselectivity?’ and ‘what would we expect in the absence of iteroselectivity?’ We did not originally answer these questions in 2014 as we used the new terminology in undisputable cases of iteroselective events where only one iteromer formed with complete selectivity. Nonetheless, it may prove useful to determine the degree of iteroselectivity, much as the concepts of enantiomeric excess or diastereomeric ratio describe the outcome of stereoselective reactions. Therefore, our recent perspective includes a method to calculate the possible outcomes of non-iteroselective reactions and introduces the concept of iteromeric excess to describes the degree of iteroselectivity.
We hope that spreading the word about the concept of iteroselectivity will help to better describe and share knowledge about iteroselective phenomena and, ultimately, develop the research around it. The concept could maybe even find some use beyond the community of organic synthesis in ways we couldn’t foresee.
References
1 J-M Lehn, Acc. Chem. Res., 1978, 11, 49 (DOI: 10.1021/ar50122a001)
2 R Lavendomme et al, J. Org. Chem., 2014, 79, 6563 (DOI: 10.1021/jo501021c)
3 R Lavendomme and I Jabin, Cell Rep. Phys. Sci., 2022, 3, 101121 (DOI: 10.1016/j.xcrp.2022.101121)
4 M Morimoto et al, Nat. Catal., 2020, 3, 969 (DOI: 10.1038/s41929-020-00528-3)










No comments yet