Helical ligands amplify solvent chirality to control reaction outcomes
As strategies for making stereodefined structures go, taking readily available chiral building blocks and building them into new molecules is essentially ideal. Unfortunately, asymmetric catalysis has not been able to take advantage of natural sources of handedness in the same way. Beyond the cinchona alkaloids and a few amino acids, we typically rely on synthetic chiral ligands or catalysts to control the stereochemical outcome of reactions, which are often generated using costly, time-consuming and often inefficient processes.
Michinori Suginome and his team at Kyoto University, Japan, have come up with a fascinating strategy using limonene – a readily available, colourless, chiral hydrocarbon extracted from peel of citrus fruits – to control the stereochemical outcome of catalytic reactions. But rather than using it as a ligand, it is used as the solvent. The chirality is then transferred through a helical macromolecular ‘helper’ to provide stereoselectivity in palladium-catalysed reactions.
While this isn’t the first report of using chiral solvents to drive enantioselective reactions, the precedent is very limited and selectivity is generally poor. Such reactions often require particular functionality in the solvent molecules, preventing the use of readily accessible and naturally occurring chiral hydrocarbon solvents such as limonene, pinene and the like. However, the team has applied the new technique to three different asymmetric reactions proceed with excellent stereocontrol, which is particularly impressive given limonene’s stereochemical simplicity.
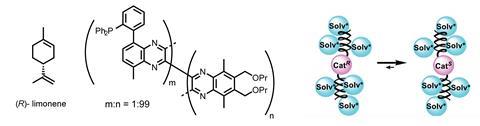
Unsurprisingly, limonene does not impart control by creating a defined chiral environment at the metal centre like a traditional ligand would. Rather, the solvent induces an achiral macromolecule to selectively form a helix of a single handedness. In turn, the helix amplifies the stereochemical influence of the solvent such that it efficiently controls the outcome of the reaction. The team built on the knowledge that poly(quinoxaline-2,3-diyl)s (figure 1) adopt single-handed helices when they have chiral side chains, but the direction of the helical induction can be modified by changing the (achiral) solvent. Extending that idea, the team formed homochiral helices from polymers with achiral sidechains by dissolving them in enantiomerically enriched limonene as solvent. Interestingly, the solvent does not even have to be enantiomerically pure, an enantiomeric excess of 65% is enough to induce completely selective helix formation.
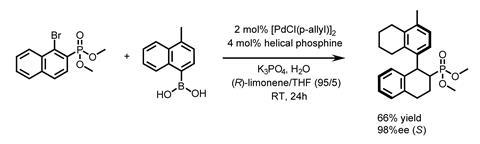
Having prepared random copolymers containing achiral side chains and phosphine ligands, the researchers first showed that using tetrahydrofuran (THF) as the solvent, these macromolecular ligands enable the coupling reaction to make a racemic binapthyl (figure 2). Changing the solvent to (R)-limonene:THF (95:5) gives the same product with a 98% enantiomeric excess and a yield of 66%, with the opposite enantiomer being synthesised simple by switching to (S)-limonene; a lot simpler than accessing both enantiomers of a complex chiral ligand. Control reactions showed that the phosphine side chain is required for the reaction to proceed, and that the helical macromolecule is crucial for asymmetric induction, supporting the premise that the solvent doesn’t directly affect the stereochemical environment at the metal centre. Once the helix is formed in the chiral solvent, it can also be isolated by precipitation with methanol. It can then mediate the reaction in an achiral solvent, to give the product with a reasonable enantiomeric excess, demonstrating memory of the screw sense.
While only a first step, this demonstration of controlling the enantioselectivity of a catalytic reaction using a naturally abundant chiral solvent is potentially a game changer, especially as chemistry must adapt and move away from fossil fuels as the predominant source of raw materials and massively increase the environmental efficiency of our industry. Extending this strategy to other catalytic reactions is likely to be challenging, with a top consideration being the stereochemical integrity of the helix at higher temperatures. That said, Suginome and his colleagues also demonstrate an asymmetric silaborative C–C bond cleavage using the very similar catalyst which gave the product in 89% yield despite a reaction time of 48 hours at 50–60°C. Perhaps there is room for optimism yet!
References
Y Nagata, R Takeda and M Suginome, ACS. Cent. Sci., 2019, 5, 1235 (DOI: 10.1021/acscentsci.9b00330)













No comments yet