From Randal Richard
I was interested to read the article by Sean McWhinnie ’Science funding in crisis’ (Chemistry World, June 2008, p40). The article had a broad sweep encompassing changes in the research assessment exercise (RAE) to research exercise framework (REF); the recent enquiry of the Innovation, universities and skills select committee into the science budget allocations; and the restructuring of the EPSRC. Having recently retired from EPSRC (initially as director of research and innovation and latterly as interim chief executive) I can correct some of the misapprehensions regarding EPSRC and to some extent all research councils in the article. Additionally having some 30 years experience in academe and research prior to joining EPSRC I have experience as both a seeker and provider of research support.
The reservations that the select committee made regarding the influence that the government appeared to have on how the science budget was allocated were repeated in the article. There was then a huge leap of logic to arrive at the conclusion that this same government influence caused the restructuring of EPSRC and redeployment of funds away from fundamental research into areas defined by the government. As far as EPSRC is concerned this conclusion is entirely wrong. The delivery plan and thus the broad future allocation of the EPSRC budget was developed whilst I was interim CEO. Preceding this there had been an 18 month strategic review during 2005-2006 and the information gathered was used in developing the delivery plan. The 6 cross-council research themes were identified by the research councils themselves. The fact that some of these themes coincide with public policy challenges identified by the government should not be surprising because they include some major scientific and technological challenges that are world wide - sustainable energy and environmental change, for example. Additionally, in 2007, in order to ensure that researchers could devote the necessary time and attention to fundamental research, EPSRC a) revised its fellowship arrangements so that the emphasis is on early and mid career researchers; b) introduced ’grand challenges’ for research; and c) established larger, longer grants.
The strategic review referred to above showed that the research environment was changing rapidly, amongst contributing factors are: the increased competition for research excellence from a wider range of nations (a factor recognised also by the US as demonstrated in the America Competes act and the publication ’Rising above the gathering storm’); increasing need for interdisciplinary approaches to research; sourcing of research and skilled staff by companies becoming increasingly global; the need to maintain an attractive research environment in the UK in terms of facilities and availability of a pool of skilled, competitive researchers to encourage inward investment to the UK research base. To cope with and embrace this change EPSRC needed a structure that was flexible, responsive and adaptable. To that end I initiated a project to develop possible models for the EPSRC that were robust but sufficiently malleable to respond to future changes. This project was internal to EPSRC, it was not suggested by the Department of Innovation, Universities and Skills (DIUS) and at no time was the project influenced by the government or DIUS officials. The models developed were handed over to the incoming CEO and the EPSRC Chairman in the last quarter of 2007 for selection, optimisation and implementation by the CEO and senior management of EPSRC. Additionally, the establishment of the Shared Service Centre and the transfer of some activities to it, all research councils will undergo some change over the next few months.
Research councils observe and support the Haldane principle in all they do and are pretty quick about reminding government and officials if they perceive the bounds are being stretched (the sharp reaction over the removal of end of year flexibility resource in March of 2007 is one example). Thus to suggest that EPSRC has bent to the (non-existent) wishes of the government is a wholly incorrect appreciation of the focus of EPSRC and its staff and their desire to work in partnership with the UK research community to provide the best environment for research within the resources available.
R W Richards CChem FRSC,
Marlborough, UK
From Roman E Sioda
I write in respect to two recent articles on quinine synthesis (J I Seeman, Angew Chem. Int. Ed. 2007, 46, 1378; A C Smith and R. Williams, Angew Chem. Int. Ed. 2008, 47, 1736) reported in Chemistry World, March 2008. After a study of the articles and the supporting information for the second one, I believe that it is highly improbable that the prescription of the synthesis in a study by Rabe and Kindler published in 1918 (Ber. Dtsch. Ges., 51, 466) could really have worked without today’s technology. This paper might be a way of protecting rights to an original sequence of synthetic reactions, which resembles the modern approach of so called one-pot synthesis. It remains, however, a mystery, why they decided to publish what they did, and not more. It seems to me that, before 1918, the proper analytical knowledge of the separation of quinine and intermediates from other reaction products, especially quinidine in the final reduction reaction, was unknown.
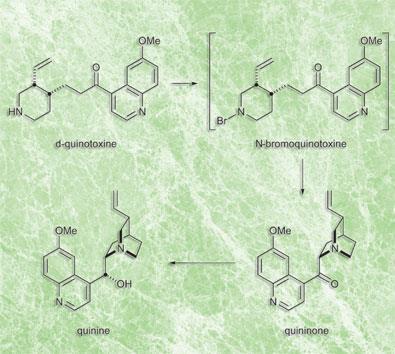
Smith and Williams’ supporting information states that a repeat of the reaction sequence performed by Thomas J Greshock, without modern separation and purification techniques, took 9 grams of substrate (quinotoxine) to yield: ’330 mg of analytically pure diquinine tartrate crystals and 268 mg analytically pure, crystalline quinine (5.9 per cent yield)’.
I believe that quinine was not actually isolated from the tartrate salt in the stated amount, because 330 mg of anhydrous salt contains 268 mg of quinine on a molecular weight basis, and some losses of quinine on L-tartrate salt decomposition by 1 M NaOH and quinine separation seem unavoidable. Anyway, the final yield of this procedure should rather be 3.0 per cent (not 5.9 per cent as above).
The described reaction sequence was performed by Smith and Williams, after it was studied in detail, in macro-scale and using all modern procedures and instrumentation. Also the know-how of the separation of quinine from quinidine, as L-tartrate salt, cited in the supporting information could not have yet been known. At that time, a successful crystallization was the main technique to separate complex reaction products.
It seems to me highly improbable that the original investigators of the 1918 paper could really have obtained quinine, if the same reaction sequence, performed with the experience gained due to all available chemical and technical laboratory knowledge anno 2007, resulted in a product with a 3 per cent yield.
I remember a remark of my former mentor, Jerzy Suszko (1889-1972), who, when I asked him how to identify an oily substance obtained from a reaction, recalled: ’Once I obtained 2 g of oily substance from a reaction, and I put it in a bottle containing 10 liters of diethyl ether. And believe me, when two years passed, the oily substance turned into beautiful crystals in the bottle’, (he might have said 2 liters instead of 10). The role of patience in separations is also illustrated by the evidence of Suszko’s graduate student R Ludwiczak?wna, who wrote about a bromination of cynchotoxine, reporting that an oily product of the reaction solidified after 3 weeks.
In Ludwiczak?wna’s paper he writes (and I translate from the Polish) ’...meanwhile the formation of bromoimine is an outstandingly capricious and unsure reaction, and during the bromination to bromotoxine, according to the scheme III to VII [III - quinotoxine and VII - quinotoxine brominated at 9-hydroxyl], one should take into account the addition of bromine to a possible unsaturated side chain. These difficulties could not be overcome by Rabe and other earlier researchers, and, because they are a crucial obstacle to the synthesis of quinine, I undertook work to overcome them.’ Ludwiczak?wna was Suszko’s first graduate student, and defended her PhD thesis in 1936.
Smith and Williams state in the supporting information that they used the commercial quinine (Aldrich), which was 90 per cent pure, without further purification. I think that this was risky and could have led to experimental errors. I had a laboratory practice (1956-1959) in the organic chemistry department at the University in Poznan, which was led by Suszko, who had defended his PhD with Rabe at the German Technical University in Prague in 1913. There, I learned that raw alkaloids were prepared for reaction by treatment with mercury acetate to separate the alkaloids from their dihydroderivatives, having an ethyl instead of a vinyl group. In the department, I was first a voluntary research assistant, and then an MSc graduate student (1958-1959) with Suszko. Initially, I had a laboratory practice in cinchotoxine research, and later did the experimental research for M.Sc. thesis on the oxidation of the vinyl group of quinidine.
In summary, the recent paper by Smith and Williams is less of a proof for the experimental work of Rabe and Kindler published in 1918, than an experimental support for the claim of the partial synthesis of quinine by R B Woodward and W E Doering in the 1940s (J. Am. Chem. Soc., 1944, 66, 849); support that has arrived rather late, but did arrive finally. The real truth, however, will probably never be known, because it will never be free of subjective opinions. Nevertheless, the discussion of the related matters may be highly enlightning.
The author thanks Prof. R M Williams for sending the supporting information.
Roman Edmund Sioda
Retired head of Department of Analytical Chemistry and Electrochemistry of Institute of Chemistry of University of Podlasie, ul. 3 Maja 54, 08-110 Siedlce, Poland.
From Norio Ise
The article by Philip Ball (Chemistry World, May 2007, p36) on colloidal interactions is misleading and biased. It argues from the authorities of Overbeek, Verwey, Landau, and Derjaguin - proponents of the DLVO theory, which has a purely repulsive electrostatic part modified by an ad hoc addition of a van der Waals short-range attraction, and explicitly equates the electrical components of the Gibbs (Ge) and Helmholtz (Fe) free energies.
We showed (N Ise and I Sogami, Structure formation in solution - ionic polymers and colloidal particles , Springer, 2005) that the DLVO model ignored the electrical osmotic factor (pe = - ? Ge/ ? V with V being system volume) remarked by Debye in his famous 1923 paper. In accordance with Debye’s reasoning, which was also followed by Fowler, Guggenheim and McQuarrie, Sogami demonstrated that, between like-charged colloidal particles, a long-range attraction exists in addition to the short-range repulsion at the Ge level.
We also pointed out that, in his criticism of the Sogami potential, Overbeek made an error in partial differentiation, which thereby invalidated his conclusion that the long-range attraction of the Sogami potential disappeared.
The purely repulsive potential ubiquitously adopted in colloid science and condensed matter physics is not axiomatically correct, due to the simplifying assumption of Ge = Fe. Furthermore, the DLVO potential does not explain the heterogeneous structure in which the colloidal particles are not in physical contact, as observed in a variety of systems. Ball fails to appreciate these specific points.
I also consider Ball’s unverified description that I am ’jubilant’ about findings by Grier’s group to be highly unprofessional. Though ignored by Ball, our disagreement with Grier’s experiments was discussed by Tata and Ise (Phys. Rev. E , 2008, 61 , 983).
Thanks are due to K Schmitz for suggestions.
N Ise MRSC
Kyoto, Japan
From Dirk Tinne
Regarding your recent story on heparin contamination ( Chemistry World China, May 2008, pC1 and Chemistry World May 2008, p20), our company - LEO Pharma Wexport Ltd, based in Cork, Ireland - manufactures heparin and low-molecular-weight (LMW) heparin (sold as innohep) and has sourced material from China in the past.
Heparin is produced from the intestinal mucosa of pigs. LEO sources its mucosa from abattoirs in Denmark, France, the UK and Ireland, and has its own equipment at each site to extract the mucosa. We are able to trace any of our heparin products from vial/syringe all the way back to the pig it came from and, if need be, demonstrate the veterinary certificate before and after slaughter.
The situation is different when it comes to sourcing from China. We have used a Chinese company in the past few years and have never experienced a problem up to now. But in two recent batches we were able to identify an impurity, before the US Food and Drug Administration and Baxter Inc. were aware of the heparin contamination problem. These batches were rejected by Wexport and no further processing took place. Subsequent batches have been clear of this contaminant.
One key difficulty is that some of these Chinese suppliers are made up of a number of workshops that are hundreds of kilometres apart. Some of these workshops have been described as ’family run operations’, with mucosa being sourced from local farmers and little or no traceability.
Another problem is that heparin is also produced by cattle and sheep, although this is not used medically. But since there is little DNA left (due to purification processes) by the time the heparin reaches its destination, it is difficult to identify the animal source.
The issue of contamination with over-sulfated chondroitin has involved pretty much everybody in Europe and the US who produces heparin and LMW heparins. LEO has carried out extensive testing of all its Heparin Sodium and innohep on the market and found it to be free of said contaminants.
D Tinne
Cork, Ireland
From Michael Russell
I noted with some dismay that the June 2008 edition of Chemistry World was accompanied by a wad of extraneous material which was substantially heavier than the magazine itself. Is it not time for the RSC to curb this obsession with paperwork? For example, did we really need a second 2008-09 year planner?
In days gone by, a single publication was all that was need to convey articles and advertisements. The current trend will prove to be expensive for the members and damaging for the environment.
M Russell CChem MRSC
Slough UK
Ed: The bumper delivery in June contained Chemistry World, RSC News(the society membership magazine), the RSC’s Annual Review, a notice of the Annual General Meeting, the latest issue of RSC Policy Bulletin, a year planner, and an advertising supplement. Chemistry World supports itself through selling advertising space on its pages, and on inserts such as the year planner - as long as it remains in profit, it receives no income from the RSC membership fee. Consequently, bulkier mailings do not necessarily increase expense for the members, and can actually save them money.
The RSC does not currently allow members to opt out of receiving some or all of the publications in the Chemistry Worldmailing. However, if you are concerned about the environmental consequences and would prefer to receive some or all of our principal publications in electronic form, please let us gauge the need for this option by contacting us via website.
From John Hudson
The possible use of commercial forests to produce biofuels (Chemistry World , June 2008, p44) would have no effect on the price of food. However, the price of paper would increase. Is that not a price rise which we would all welcome?
J A Hudson CChem FRSC,
Loweswater, Cumbria, UK
From Jeff Leigh
Derek Lowe’s discussion (Chemistry World , June 2008, p20) of the mutual incomprehension of chemists and biologists reflects a common but not inevitable situation. I may not be completely objective in such matters, but the mutual understanding Lowe desires is precisely what we achieved at the late, lamented Unit of Nitrogen Fixation at the University of Sussex.
Our remit was to discover the mechanism of biological nitrogen fixation. We were organised into two groups, chemists and biologists, each imaginatively and understandingly led by prominent scientists. However, from the beginning we were encouraged to work as an inter-disciplinary group and we were regularly and officially judged as a whole. We were forced to interact with each other.
Every one of some 15 senior scientists, ranging in expertise from inorganic chemists through biochemists and microbial geneticists to microbiologists, as well as all the technical support staff, were expected to attend all the seminars, and to discuss our six-monthly progress reports together. Mutual understanding was not achieved rapidly, or without some grumbles from recalcitrant individuals, but it was achieved.
The Unit of Nitrogen Fixation became the world’s leading laboratory in the study of biological nitrogen fixation. Visitors came from all over the world to learn from it. It was invariably the major source of contributions to international conferences on nitrogen fixation.
The mechanism of biological nitrogen fixation is still incompletely understood. The sponsors of the unit eventually decided that the economic rewards of 25 years of research did not justify the expense of continuing the work. However, the academic returns from this expenditure were enormous. The unit was an example of what Derek Lowe would like to see.
An organisation such as the unit requires at the least a funding body that is prepared to support a clearly defined project of sufficient size and projected life. That is unlikely today. In addition, the current pattern of university organisation, at least in the UK, where individual researchers pursue their own research in order to forward their individual careers, strongly militates against large cooperative research undertakings.
If research groups such as the unit are desired, and I think they should be, then a considerable reorganisation of academic funding and career structures would seem to be called for.
J Leigh
Brighton, UK











No comments yet