A distillation method that came out in the wash
Much of chemistry is taught in metaphors: electron clouds, energy flows, close-packed spheres, reaction landscapes and flipping magnets. These pictures, while embedded into a deeper theoretical structure, provide mental shortcuts that help make predictions, formulate experiments and cement understanding. And yet, danger lurks in such ideas; they can also prevent us from seeing things that might otherwise be obvious. As the biologist and cybernetics guru Norbert Weiner wrote so pithily, ‘The price of metaphor is eternal vigilance’.
This phrase came to my mind when I was trying to make sense of the strange lineage of apparatus that I first saw in the stores of the Science Museum in London. Early in the 19th century, chemical distillation underwent a transition, driven by the need to separate members of the homologous series of organic compounds. Small differences in boiling temperature between, say, butyl and amyl alcohol meant that the use of a traditional retort (a bent, long-necked flask) required multiple distillations to obtain pure material. When Adolphe Wurtz introduced his ‘tube à bulles’ (bubble tube) one-shot distillations with good separation became standard.
But how did it work? There was little real understanding: key concepts like equilibrium, vapour pressure, temperature and energy were still, at best, in their infancy. Distillation theory was based around the rise of the lighter ethereal vapour and the descent of the wet ‘phlegm’. The spirits industry described the process as ‘washing’; what today we would call fractionation was called ‘dephlegmation’. In the 1820s the French–Belgian still designer Jean-Baptiste Cellier-Blumenthal mashed up several designs to create the first highly efficient continuous still with bubble trays, horizontal platforms arranged in stacks where the vapour bubbled its way through the descending wash.
Eduard Linnemann (1841–1886)
The difference would be spotted by Eduard Linnemann. Born in Frankfurt am Main, he studied chemistry in Heidelberg, taught by Robert Bunsen and August Kekulé. Linnemann followed Kekulé to Ghent as his assistant before heading to Lemberg in Galicia (today Lviv, Ukraine) to become assistant to another ex-Heidelberg academic, Leopold von Pebal. He got lucky. Just as Linnemann secured his habilitation, von Pebal ‘received the call’ from the University of Graz and decamped, leaving Linnemann to slide seamlessly into his place in 1865. He was soon full professor.
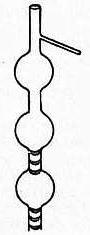
Throughout this time, Linnemann had been working on homologous series, publishing boiling temperatures and helping to reinforce the structural theory of chemistry. In 1871, he unveiled a new design of fractionator. His paper reveals a hint of insecurity, observing that laboratory distillation lagged far behind industry. In industrial installations ‘a kind of washing’ takes place because the vapour is ‘compressed’ and ‘forced’ to bubble through the liquid. This ‘washing’ is not possible in a simple or even Wurtz distillation. He therefore proposed a new fractionator that combined the two approaches: little baskets of platinum mesh inserted at intervals in the tube to collect the liquid, making ‘washing’ possible. Furthermore, as flames were used for heating, superheated vapour never reached the thermometer, yielding more accurate boiling temperatures.
Linnemann’s paper was widely read and his method was adopted in textbooks of organic chemistry, including Ludwig Gattermann’s. Yet when our glassblower, John Cowley, built one for me a couple of years ago with little copper mesh baskets, the results were rather maddening – the baskets filled with liquid and the fractionator tended to belch liquid upwards unless the flask was heated extremely slowly. This flooding issue was well known and spurred the development of several dozen designs over the next 40 years, sporting little funnels, glass loops and channels. All but one has disappeared: only the Snyder column survives, used with the Kuderna-Danish pesticide residue concentrator. Its glass beads serve to create pools of liquid that prevent the analyte escaping with solvent aerosol.
But for Linnemann there was also trauma: Galicia was granted increasing autonomy and the university was ‘polonised’. He lost his post, moving first to Brünn (today Brno in the Czech Republic) and then to Prague. His interests shifted to the search for new rare earth elements. Though increasingly ill he continued to work in the lab. While analysing the mineral orthite, a silicate with a peculiar composition, he observed new lines in the flame spectrum of an acid extract. Convinced that he had discovered a new element, he wrote a paper on his deathbed announcing the discovery of ‘austrium’. It was not to be. Months after his death, the Austrian chemist Richard Pribram and Paul-Émile Lecoq de Boisbaudran, the French element hunter-extraordinaire, showed the spectral lines to correspond to those of one of Lecoq’s own elements, gallium. Linnemann’s name would fade into obscurity.
Was Linnemann’s thinking trapped by the seductively simple idea of ‘washing’? That suspicion makes me very nervous. How many deeply embedded metaphors prevent us from seeing things that are deep and important?
Acknowledgments
I am grateful to Talitha Humphrey who tested Linnemann’s and other columns and began to exhume his story. Rupert Cole also invited me into the Science Museum stores and Philip Ball put Norbert Weiner on my map.

















No comments yet