Lithium-ion batteries are the gold standard rechargeable batteries in consumer electronics such as mobile phones because they’re light and hold plenty of energy. For more demanding applications, such as electric vehicles, however, researchers want batteries that hold more energy, letting cars go further on a single charge. To do this, electrical engineers need a better anode, but many experimental designs expand and then crack when charged repeatedly. Now, US researchers have created battery anodes that heal themselves after they fracture, substantially prolonging battery life.
The current material of choice for anodes is graphite. When the battery is charged, lithium cations intercalate themselves between the planes of carbon, forming LiC6. These are released during discharge. For the battery to hold more charge, the anode needs to hold more lithium. Silicon is a promising material as it forms either Li15Si4 or Li21Si5. Inevitably, though, all those extra atoms forcing themselves into the anode makes the silicon expand by up to 300%, which can cause it to shatter in just a few charge/discharge cycles.
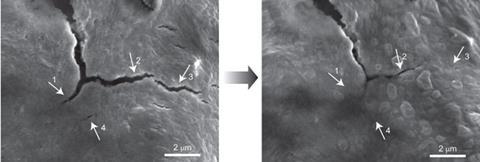
Yi Cui's and Zhenan Bao's groups at Stanford University have joined forces to produce an elegant solution to anode fractures. Cui's group has previously worked on lithium-ion batteries, while Bao's has designed conductive, self-healing polymers for synthetic skin and other applications. To tackle this battery problem they embedded silicon microparticles inside a randomly branched, hydrogen bonding, amorphous polymer.
When the silicon microparticle anode start to expand during use, the polymer chains stretch out and rearrange themselves, accommodating expansion without shattering. If a tear does occur, the hydrogen bonding at the crack interfaces lets it heal. Embedded carbon black particles gave the polymer electrical conductivity.
The team tested anodes made from the material by repeatedly charging and discharging electrochemical cells that used them. After 90 cycles, the electrodes retained 80% of their original discharge capacity. An anode that embedded the silicon microparticles in a seaweed gel retained only 47% of its capacity after 20 cycles. Anodes using other polymer binders performed even worse.
The team is now optimising its lithium–polymer composite to improve the cycling stability and other properties. ‘Several hundred cycles are needed for cell phone batteries and several thousand for electric vehicles,’ says Bao. ‘We are trying to understand the detailed chemical and physical processes to have a better idea of what parameters of our self-healing polymers we need to change.’
Materials scientist Nancy Sottos of the University of Illinois, Urbana-Champaign, US, whose research group uses a different type of self-healing polymer for battery anodes, says: ‘It is a very good approach for the system that they're looking at. What isn't clear is whether this will work if you have a high filler content [the silicon in this case] and maybe less polymer, which is more typical of commercial electrodes.’






No comments yet