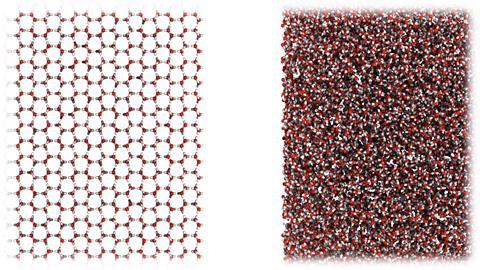
Medium-density amorphous ice has a structure and density similar to liquid water
Water keeps getting stranger. To the many forms of ice already known, researchers at University College London (UCL), UK, have now added one more.1 The new phase particularly complicates the picture because it is not crystalline but amorphous and, with a similar density to water itself, looks rather like a frozen snapshot of the liquid state. That contrasts with water’s peculiar expansion when it freezes into regular ice (denoted ice Ih, with a hexagonal structure). The discovery throws a cat among the pigeons for the conventional view of how liquid water is related to its frozen forms.
It already seemed absurdly profligate of nature to give the deceptively simple H2O molecule at least 22 ways to arrange itself in the solid state, depending on the conditions of temperature and pressure. Changing these parameters alters the balance of enthalpies (due to intermolecular bonding) and entropies (due to ordering) associated with different configurations – but that many possibilities suggests a remarkably delicate balance of such factors. This protean nature lies with water’s almost unique propensity to form highly interconnected three-dimensional networks of hydrogen bonds – four bonds for each molecule (or more, if a hydrogen bond bifurcates at a single locus). Hydrogen bonding is an enthalpic win but, by creating local ordering, an entropic loss. What’s more, because hydrogen bonds keep the molecules at arm’s length, so to speak, they sacrifice the favourable energetics offered by non-directional van der Waals attractions between molecules. When water freezes to ice Ih, the result is then a beautifully ordered arrangement in which the molecules form a network of six-membered rings – but with a lot of empty space in their centre, accounting for the lowered density. Squeeze the solid and the empty space may get filled, either by bending hydrogen bonds (which weakens them) or more profound restructuring of the entire lattice.
Competing powers
This balance of factors partly accounts for the complexity of biomolecular hydration, where water might be restructured in subtle ways by the geometric and chemical constraints imposed by biological molecules. The balance between factors that favour high and low density is also reflected in the existence of two previously known amorphous forms of ice, made by freezing the compound in non-standard ways – for example, by vapour deposition of water onto a cold surface. One of these, unimaginatively called low-density amorphous (LDA) ice, reflects the dominance of the hydrogen bond’s ‘social distancing’ influence, while in high-density amorphous (HDA) ice, pressure encourages more intimate molecular approaches. It is now widely suspected that these two forms of ice are the counterparts of two distinct liquid phases (high- and low-density liquid, HDL and LDL), born of a similar competition of factors, that may exist in the highly supercooled region of water’s phase diagram.
This balance of factors partly accounts for the complexity of biomolecular hydration, where water might be restructured in subtle ways by the geometric and chemical constraints imposed by biological molecules. The balance between factors that favour high and low density is also reflected in the existence of two previously known amorphous forms of ice, made by freezing the compound in non-standard ways – for example, by vapour deposition of water onto a cold surface. One of these, unimaginatively called low-density amorphous (LDA) ice, reflects the dominance of the hydrogen bond’s ‘social distancing’ influence, while in high-density amorphous (HDA) ice, pressure encourages more intimate molecular approaches. It is now widely suspected that these two forms of ice are the counterparts of two distinct liquid phases (high- and low-density liquid, HDL and LDL), born of a similar competition of factors, that may exist in the highly supercooled region of water’s phase diagram. This region is extremely hard to access experimentally, but experimental and computational studies now support the notion of a first-order (abrupt) phase transition between HDL and LDL, ending at a critical point like that between conventional liquid and gas phases.2–5

This tidy story might need modification – or more – in the light of the new findings by Christoph Salzmann and his colleagues at UCL. They have found that ball milling of ice Ih – grinding it up with churning steel ball-bearings – at 77K converts it gradually into a new form of amorphous ice with a density of 1.06g/cm3, intermediate between HDA and LDA and very close to that of liquid water itself. The researchers show that this is a genuine new phase, apparently formed by repeated shearing of ice Ih, which can be recrystallised to Ih (via a defect-ridden form) when warmed – a substantial exothermic process. They call the new phase medium-density amorphous (MDA) ice.
What’s the relationship between MDA and liquid water? There may be none – MDA might be merely a heavily disrupted form of Ih unconnected to the liquid. The similarity of densities might then be just coincidence. Alternatively it could be a genuine frozen form of the liquid, contrary to the prevailing view that water can’t freeze without finding a new accommodation of the various factors dictating its structure. Both the density and the structure (as deduced from computer simulations and characterised by the first sharp X-ray diffraction peak) of MDA look rather similar to that of the liquid. It might then be that MDA represents a true glassy state of liquid water that is only ever metastable relative to HDA and LDA.
At any event, MDA ice produced by shear could perhaps form in the frigid interiors of the icy moons of gas-giant planets like Jupiter, where normal ice at very low temperatures is wracked by the gravitational influence of the planet. If so, the considerable release of energy if MDA recrystallises could affect the energy budget of the planetary interiors. That’s the thing about water: for all its peculiarities, it is ubiquitous in the universe – and so its quirks often have wide, even cosmic implications.
References
1 A Rosu-Finsen et al., Science, 2023, 379, 474 (DOI: 10.1126/science.abq2105)
2 Y Suzuki, Proc. Natl. Acad. Sci. USA, 2022, 119, e2113411119 (DOI: 10.1073/pnas.2113411119)
3 K-i Murata and H Tanaka, Nat. Mater., 2012, 11, 436 (DOI: 10.1038/nmat3271)
4 S Woutersen et al, Science, 2018, 359, 1127 (DOI: 10.1126/science.aao7049)
5 T E Gartner III et al, Phys. Rev. Lett., 2022, 129, 255702 (DOI: 10.1103/PhysRevLett.129.255702)












No comments yet