The efficiency of solar cells made using perovskite semiconductors has risen meteorically. Will their trajectory take them from research to industry, asks Andy Extance?

When Henry Snaith saw how easy it was to make hybrid organic–inorganic perovskite crystals, he was hooked. The solar cell researcher, based at the University of Oxford in the UK, had been struggling to make light-absorbing quantum dots, conducting three-day-long syntheses yielding just a few milligrams. Then, in 2008, Snaith saw Tsutomu ‘Tom’ Miyasaka from Toin University of Yokohama, Japan, present dye-sensitised solar cells (DSSCs) like he was studying, but using perovskite materials as the light-absorbing component. ‘He had a nice video where, after mixing two salts, he spin-coats a solution on a film and while drying it crystallises the perovskite,’ Snaith recalls. ‘The ease of making perovskites is very attractive.’
Though Miyasaka’s cell delivered a modest sunlight-to-electricity conversion efficiency of 3.8%, it ignited the hottest area in solar cell research today.1 As well as perovskites’ simple synthesis, scientists are discovering that they also boast unique structural and electronic properties. Hundreds of papers have followed, and efficiencies have leapt forward, with research devices now challenging commercial solar cells. Though photovoltaic technologies are notoriously susceptible to hype, few have attracted so much attention and optimism. But with technical and commercial hurdles yet to clear, will perovskites satisfy these expectations?
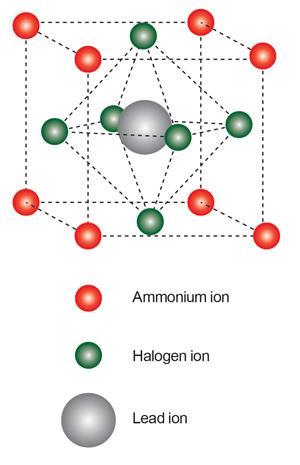
Although there is a calcium titanate mineral called perovskite, the name also refers to all materials sharing its ABX3 crystal structure. Miyasaka brought perovskites to DSSCs thanks to his PhD student Akihiro Kojima, who studied their potential as semiconductor materials for light-emitting diodes during his master’s degree. Their best initial perovskite was the organic–inorganic hybrid methylammonium lead triiodide, CH3 NH3 PbI3, made by mixing methylammonium iodide, CH3 NH3 I, and lead(ii) iodide, PbI2.
Conducting inquiries
Miyasaka’s team spin-coated the perovskite onto nanocrystalline titania (TiO2) paste that served as their DSSC’s anode, collecting electrons generated by the light-absorbing perovskite (see Perovskite photovoltaic basics). Despite handling difficulties, that system provided an alternative liquid electrolyte DSSC with strong light absorption power, but also posed a question: just why was it so good? ‘Perovskites were first considered to work as quantum-dot-type sensitisers because their light absorbing wavelength is tuneable,’ Miyasaka explains.
After the Toin University team published its first paper, Snaith asked Miyasaka if his PhD student Mike Lee could visit Japan to learn perovskite synthesis. That 2011 visit would move the materials away from liquid DSSCs, whose use of volatile liquids that need careful containment is a major drawback. ‘He solidified our original perovskite-based cell using a solid-state organic hole conductor,’ Miyasaka says. ‘Mike went back to Oxford and one year later succeeded in fabricating a high-efficiency cell.’2
The high efficiency arose when Snaith and Lee swapped the conductive titania layer for insulating alumina (Al2 O3). ‘In cells made with TiO2, charge seemed to be transported and collected faster than with organic dye sensitisers,’ says Snaith. ‘We postulated that the perovskite was also conducting some charge, so we made dummy alumina cells to see. It was quite a surprise when the first cells were over 10% efficiency, when they were only 7% using TiO2.’ The unexpected result forced a rethink: rather than quantum dots, it became clear that perovskites formed sheet-like semiconductor crystals on porous scaffolds such as titania and alumina.
Perovskite photovoltaic basics
When a photovoltaic cell absorbs light, a quasiparticle known as an exciton typically forms in its electronic structure. The exciton comprises an electron that has been nudged into a higher energy level by an incoming photon and the positively charged hole it leaves behind. The electrons and holes are both charge carriers that move towards a cell’s anode and cathode respectively, often through electron- and hole-transport materials when connected into a circuit. If they reach their electrodes without the photon’s energy being lost as heat due to interactions with defects in the crystal, for example, an electric current is generated.
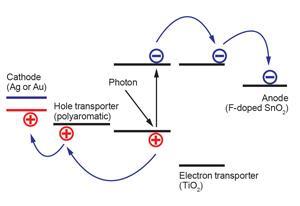
In perovskite solar cells, light passes typically through a transparent fluorine-doped tin oxide anode and titania electron-transport layers, forming excitons in the photosensitive perovskite material. Electrons move through the titania towards the anode, while holes move through a transport material, usually a polyaromatic, to a gold or silver cathode. Part of perovskite materials’ success comes because, unlike conventional absorbers in dye-sensitised solar cells, they are also semiconductors that can transport charges themselves. It’s therefore possible to minimise and even eliminate the transport layers, meaning cell designs are evolving rapidly.
A tale of two talks
Meanwhile, at the Swiss Federal Polytechnic in Lausanne (EPFL) Miyasaka’s initial paper had also alerted DSSC co-inventor Michael Grätzel to perovskites’ potential. He was attracted by their strong light absorption compared to other sensitisers, but in his team’s attempts to reproduce the findings, they found the perovskites highly unstable. Then, in 2011, Grätzel met Nam-Gyu Park from Sungkyunkwan University in South Korea, who had boosted perovskite-based DSSCs’ stability and efficiency, partly by switching spin-coating solvent from N,N- dimethylformamide to gamma-butyrolactone.3
There’s friendly competition on several fronts
Michael Grätzel
To exploit this, Grätzel and Park teamed up to make a 9% efficiency solid-state cell, using the same organic hole conductor as Lee, but retaining the titania scaffold.4 ‘We sent the paper in for publication and then Henry Snaith gave us a talk and we discovered we’d been working on very similar things,’ Grätzel remembers. Submitted after but published before their rivals’ paper, the solid composition of these ‘mesoscopic’ cells – whose name reflects the 5–50nm pore size of the scaffold – further boosts their stability.
Grätzel and Snaith have since been spearheading largely separate explorations of the solar cell architectures that perovskites enable. For their cell architectures, Grätzel and collaborators have mainly stuck with mesoporous metal oxides, while Snaith, who’d previously worked in Grätzel’s lab, has moved away from them. ‘There’s friendly competition on several fronts,’ Grätzel chuckles. ‘Who has the best cells? What’s the best configuration? Who will make it to industry?’
Snaith first started thinning down his alumina scaffold, and then removed it entirely to give planar, thin-film cells. Other thin-film photovoltaic materials are usually simple to produce but amorphous and therefore riddled with defects that handicap their performance relative to slow-to-make highly crystalline silicon cells. Thin-film perovskites, by contrast, are highly crystalline and easy to make.
‘The fact that you just mix two salts and the product crystallises and forms a near-perfect semiconductor is a total surprise to everyone,’ Snaith enthuses. ‘That’s really the exciting aspect of it. Perovskites have all the processing advantages of amorphous materials, but appear to have the high-quality semiconductor nature of highly crystalline semiconductors. I don’t believe there’s another family of materials that has these properties.’
From spin-coating to spin-out
The spin-out company that Snaith founded, Oxford PV, is now trying to capitalise on this behaviour, developing large-area solution-coating methods. Rather than try to compete with existing commercial technologies, their first target is to produce semi-transparent cells that could turn windows into power stations. ‘For building-integrated PV, there’s a very good market for lower efficiency if it’s got an aesthetic advantage,’ Snaith says.

Yet for all the rapid progress that has been made so far, many aspects of perovskites’ desirable characteristics remain mysterious. Working closely with Snaith, Annamaria Petrozza from the Italian Institute of Technology in Milan has shown that high-quality crystals help explain many of them. She highlights new synthesis methods published last year, the one Snaith’s team introduced to make their thin-film cell5 and another from Grätzel’s team,6 which pushed efficiency beyond 15%.
‘Their main demonstration was that if you control the film morphology you’re able to make a big jump in device efficiency,’ Petrozza says. ‘The organic cation has more of a mechanical function – it doesn’t directly contribute to the electronic properties of the materials. The electronic properties are in principle governed by the inorganic agents, the lead and the halides. However, the size of the organic cation is able to induce stress or confinement effects that can then determine the optoelectronic properties of the material itself. Then, how the crystals self-assemble can definitely affect the way charges are transported and light absorbed.’
Since their first collaboration with Miyasaka, Snaith’s team has normally used mixed iodide–chloride perovskites, which provide an important boost.7 ‘A small amount of chlorine doping induces a much longer diffusion length for the generated carriers, for example, but how this is happening is not clear yet,’ Petrozza says. However, the chlorine atoms may not be participating in the perovskites’ structure as much as has been thought, she adds.
Solar sells
Long diffusion lengths mean more electrons and holes can reach perovskite cell electrodes and create an external electrical current. They also help build up high charge densities within cells, in turn determining ‘open-circuit’ voltages, the electrical power a cell generates and ultimately its efficiency.
Perovskite is very, very cheap
Tsutomu Miyasaka
‘The highest open-circuit voltage a photovoltaic system has ever achieved for a solar cell absorbing all visible light up to wavelengths of 800nm was in GaAs, which is capable of generating 1.12V,’ Tsutomu explains. He says his team is about to report a solar cell whose output voltage is maximised to the theoretical limit. Rather than the thin-film architecture Snaith’s group is pursuing, the device brings together a high-quality perovskite crystalline film and a crystalline organic hole transport material. ‘A fully crystalline solar cell made by this junction generates 1.21V, which is the largest voltage ever obtained,’ Miyasaka says.
This performance need not come at a premium either. ‘Perovskite, made of lead and organic halides, is very, very cheap,’ says Miyasaka. ‘300–400nm thick perovskite only weighs 1.2–1.7g/m2. Based on the price of mass-produced perovskite, that costs $1 per m2 [£0.60 per m2].’ Adding in other materials, the engineer estimates overall costs at $25–30 per m2, less than silicon or CdTe cells. ‘In addition, production cost is much cheaper for perovskite than for silicon because it’s a rapid solution process, free of high temperature and vacuum.’
Speed isn’t everything
After independently developing their own perovskite cells, Sang Il Seok’s team at the Korea Research Institute of Chemical Technology (KRICT) in Daejeon has collaborated closely with Grätzel’s on mesoscopic designs. Together, Grätzel and Seok’s teams found they could increase their open-circuit voltage by swapping the organic hole conductor in their cells from the previously used spirobifluorene polyaromatic ring known as spiro -OMeTAD to a poly-triarylamine.8 In doing so, they delivered 12% efficiency, a record for perovskite cells at the time.
Seok has also developed spin coating to give the high-quality crystals needed for the best efficiencies, by using gamma-butyrolactone and dimethylsulfoxide (DMSO) as solvents. The benefit comes because, ironically, perovskites’ attractively rapid synthesis can be too fast. If they form as coating solvents are boiling off, their crystal structures can be disrupted. ‘Even though we use high boiling point solvents, they can evaporate during spin coating due to the centrifugal force,’ Seok says. DMSO helps avoid the damage that would cause by preventing crystal formation until spin-coating has stopped. ‘It acts as both solvent and reactant – it can retard the reaction between lead iodide and methylammonium iodide.’
The jury is still out
Michael Grätzel
And now, the KRICT team has incorporated a planar thin-film layer into the mesoscopic devices it produces using these methods.9 In doing so it has gained the highest certified efficiency for any perovskite solar cell, at 17.9%. This enters the range of commercial photovoltaic module efficiency records, which stand at 17% for CdTe and 21.5% for silicon, and avoids a problem plaguing planar perovskite devices.
The problem – hysteresis – is most obvious in cell efficiency measurement, potentially bringing performance claims into question. A device is typically first scanned from high voltage to low, but if a reverse scan is then immediately conducted, planar cells often show a different profile. ‘We can’t report this data because well-defined solar cells shouldn’t show large hysteresis,’ Seok admits. ‘To solve that problem we spent a lot of time controlling the architecture and the thickness of perovskite layer and the pore width. Finally, we settled on a bilayer, combining a mesoscopic and a planar layer.’
Can hope trump hype?
One problem facing the burgeoning field rests at the very centre of these high-quality crystals – poisonous lead. ‘The issue is that it’s a soluble compound,’ Grätzel explains. ‘We have lead batteries and CdTe solar cells, which could be much more problematic, but they’re not soluble. Industry will be very careful about deploying a material like that on a large scale.’
Grätzel is confident that this obstacle will be overcome, if not by solar module design, then by tin perovskites developed by Snaith’s team and others.10 As well as eliminating toxicity, they could alternatively enable tandem cells that boost efficiency further by having two different perovskite layers absorbing different parts of the solar spectrum. Grätzel highlights that tandem designs are already attracting ‘big interest from the silicon community’, who want to incorporate perovskites into their devices.
As demonstrated by Miyasaka’s very first cells, perovskites’ solubility also translates to instability on exposure to moisture. This could be another problem for large-scale, long-term solar power generation, although Grätzel explains that his group has recently made important progress.11 ‘We address stability with a hole-conductor-free system that uses carbon as a collector electrode,’ Grätzel explains. ‘You get cells that are stable for 1000 hours under full sunlight and at 45°C.’ Yet industry standard tests are done at 85°C, 85% humidity, and he admits that, under those conditions, ‘the jury is still out’.
Perovskite cells’ promise is great, but the challenges that remain pose no small risk either, Grätzel warns. ‘One should refrain from overstating the case, even though it’s undoubtedly very positive,’ he stresses. ‘There are a lot of questions and pitfalls and we have to be very careful to not run into a wall. But this is a marvellous class of materials, and the improvements have been astonishing. It’s been an amazing ride.’
Andy Extance is a science writer based in Exeter, UK
References
1 A Kojima et al, J. Am. Chem. Soc., 2009, 131, 6050 (DOI: 10.1021/ja809598r)
2 M M Lee et al, Science, 2012, 338, 643 (DOI: 10.1126/science.1228604)
3 J-H Im et al, Nanoscale, 2011,3, 4088 (DOI: 10.1039/c1nr10867k)
4 H-S Kim et al, Sci. Rep., 2012, 2, 591 (DOI: 10.1038/srep00591)
5 M Liu, M B Johnston and H J Snaith, Nature, 2013, 501, 395 (DOI: 10.1038/nature12509)
6 J Burschka et al, Nature, 2013, 499, 316 (DOI: 10.1038/nature12340)
7 S D Stranks et al, Science, 2013, 342, 341 (DOI: 10.1126/science.1243982)
8 J H Heo et al, Nat. Photon., 2013, 7, 486 (DOI: 10.1038/nphoton.2013.80)
9 N J Jeon et al, Nat. Mater., 2014, DOI: 10.1038/nmat4014
10 N K Noel et al, Energy Environ. Sci., 2014, DOI: 10.1039/c4ee01076k
11 A Mei et al, Science, 2014, DOI: 10.1126/science.1254763










No comments yet