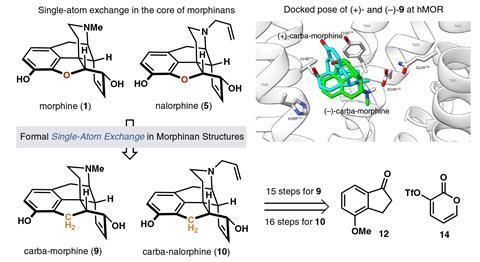
A single atom swap in the core structure of morphine has created an analogue with the opioid’s signature painkilling activity but without the problematic side effects. The O-to-CH2 exchange removed a key hydrogen-bonding interaction in the binding pocket, activating the receptor to dull pain while suppressing the secondary pathways responsible for addiction and respiratory depression. This pharmacological profile of what has been dubbed carbamorphine suggests similar core modifications could help to design safer opioids, say the team behind the work.
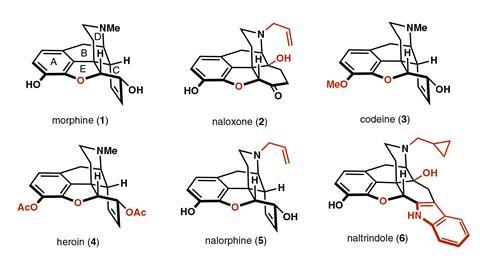
The analgesic activity of morphine has been exploited by humans for thousands of years and opioids are still some of the most prescribed medicines today. ‘Morphine is an agonist of the µ-opioid receptor, which means that it activates the receptor, recruits proteins, and ultimately reduces the transmission of pain signals [and] leads to the production of dopamine in the brain,’ explains Richmond Sarpong, a synthetic chemist at the University of California Berkeley. However, concomitant activation of other pathways – such as the recruitment of beta-arrestin 2 proteins, which mediate tolerance and dependence – also induces worrying side effects including reduced respiratory activity and addictive behaviours.
Peripheral modifications to morphine’s structure can modulate this activity – doubly acetylated heroin is significantly more addictive, while n-allylated nalorphine acts as an antagonist to reverse the effects of overdose. However, the influence of changes to the core pentacyclic framework remain unknown.
Modelling studies have previously identified a hydrogen-bonding interaction between the furan E-ring oxygen and a tyrosine residue in the receptor and, for Sarpong, this posed a tantalising question. ‘I wondered what would happen if one removed [that] bond,’ he says. ‘We decided [to swap] the E-ring oxygen for another atom that will not hydrogen bond. We settled on carbon and so we decided to install a methylene group.’
Rebuilding morphine
A single atom skeletal edit directly to the morphine framework is currently beyond the scope of chemistry. However, armed with the synthetic logic developed during previous successful syntheses (notably the cephanolide family in 2022), the team devised an efficient 15-step route to the carba-analogue, employing an intramolecular cycloaddition to form the pentacyclic core. The racemic product was readily separated into its component enantiomers using chiral chromatography and the sequence also provided easy access to carbacodeine and carbanalorphine for future biological studies. ‘Overall, our synthesis compares favourably (from a step count perspective) with existing syntheses of morphine,’ Sarpong says. ‘Importantly, our synthesis is scalable to allow us to access hundreds of milligrams in one pass.’
The team then began to explore the analogues’ pharmacological activity. Intriguingly, unlike morphine for which only the (-)-enantiomer is active, both (+)- and (-)-carbamorphine demonstrated good binding affinity with the µ-opioid receptor in cell assays and comparable, though slightly reduced, potency at therapeutic doses. Most notably, when trialled in mice, (+)-carbamorphine did not reduce breathing rate or create addictive behaviour patterns, addressing two of the most concerning side effects of morphine use.
These promising preliminary results suggest core atom modifications could become an important tool in the design of safer and more specific opioid medications but carbamorphine itself is still a long way from clinical trials. ‘To even start to consider this possibility, we will need to develop a shorter synthesis to make larger quantities of these materials. In addition, we will need even more rigorous in vitro and in vivo assays, not only on carbamorphine and carbanalorphine, but also on their metabolites, which would need to be fully characterised,’ explains Sarpong.
Twisted tale
Focusing instead on the underlying mechanism behind carbamorphine’s contrasting biological profile, they conducted docking and simulation studies to model the binding behaviour. These computations revealed that (+)-carbamorphine occupies the same binding pocket as (-)-morphine but is significantly twisted relative to the natural compound. This rotation reduces the number of interactions with the receptor and induces a steric clash with key residues on the opposite side of pocket, which the team propose may explain (+)-carbamorphine’s moderate potency and suppression of side effects.
‘There’s a lot of exciting data in this paper and, because they have such detailed docking and proposed binding models, I think they’re in a really good spot to think about further optimising these compounds to increase potency and selectivity,’ says Joshua Pierce, a synthetic chemist at North Carolina State University. ‘I think it would be interesting to couple this modification with some of the known derivatives of morphine to see whether it could be further enhanced and differentiated from these compounds.’
For both Sarpong and Pierce it is this conceptual approach, rather than the analogue compounds themselves, which marks the most significant advance at this stage. ‘We have demonstrated that making single core atom changes to the morphine framework leads to a novel (and unanticipated) biological profile and so as a community, we should be looking to make more of these types of changes to molecules and not just focusing on the periphery of compounds,’ says Sarpong.
While we are continuing to explore the effects of these modifications on morphine, developing synthetic methods to streamline this process is equally important, he adds. ‘We would like to be able to make single-atom changes directly on the core of compounds like morphine. Access to this type of technology would allow us to swap out the E-ring oxygen for not only carbon, but nitrogen, sulfur, silicon, etc.’
References
S Akiyama et al, Proc. Natl. Acad. Sci. USA, 2025, DOI: 10.1073/pnas.2425438122
















No comments yet