By unpicking how cephalopods change their looks to match their environment, researchers are aiming to reverse-engineer a host of novel materials. Emma Davies reports

Alon Gorodetsky remembers the exact moment when his obsession with the camouflage systems of squid and cuttlefish began. A little over three years ago, he attended a self-assembly meeting and happened upon a presentation by Roger Hanlon, a marine biologist from the Woods Hole Marine Biological Laboratory, US.
Hanlon showed breathtaking videos of cephalopods performing amazing feats of camouflage and spoke about exciting applications for manmade camouflage technology. ‘It just blew me away and I thought: I have to work on this,’ recalls Gorodetsky.
Back with his biomolecular electronics group at the University of California, Irvine, in the US, Gorodetsky soon built up cephalopod-inspired research projects looking at both conductive materials and camouflage that responds to external stimuli. At the heart of the projects sit reflectins, a remarkably robust family of proteins that play a key role in cephalopod camouflage. As a materials scientist, what really grabbed Gorodetsky’s attention was the fact that reflectin can be processed and worked with, ‘essentially like a polymer’.
His team has already used films of reflectin to build camouflage coatings. Using acetic acid, they can tune the reflectance of the coatings over more than 600nm, so that they effectively disappear and reappear when seen through an infrared imaging camera, as used by the military during night patrols.1 These films, they say, are a crucial first step towards developing reconfigurable biomimetic camouflage technologies for stealth applications.
The researchers have also shown that reflectin can conduct protons just as well as state-of-the-art manmade proton conductors, which are central to renewable energy and bioelectronic technologies ranging from fuel cells to transistors. They have made durable and effective protonic devices using the reflectin films that they had designed earlier for camouflage.2
Gorodetsky is so taken with cephalopods that he often thinks it would be nice to install a tank containing a small squid in his office so that he can observe the camouflage process as different patterns are inserted as a backdrop. At Woods Hole, this is exactly what Hanlon has spent decades doing, although he still dives to study cephalopods in their natural environment too. In the lab, Hanlon studies the minute, complex structures that make up the camouflage layers in cephalopod skin using a ‘heck of a lot’ of microscopy and spectroscopy.
Seeing the light
Hanlon’s camouflage videos are so entrancing that Gorodetsky still watches them almost every day. ‘Squid are real-life shape shifters,’ he enthuses. ‘What’s amazing is that they don’t have eyes in the back of their head but can still imitate what is behind them. It’s the equivalent of you backing up to a wall and turning into a filing cabinet,’ he jokes. What’s even more amazing is the fact that most cephalopods are colour blind, and how they therefore achieve such a colourful camouflage remains an interesting biological question.
They have a highly distributed nervous system, which means that processes do not necessarily need to go through the brain. And recent groundbreaking research by Hanlon identified light-sensitive proteins called opsins, normally found in photosensitive cells in the retina, all over cephalopod skin. These opsins could have a role in helping cephalopods to sense their multicoloured environment. ‘They may be for colour, but they may just be for brightness. We have not been able to prove functionality,’ says Hanlon. ‘It’s extremely difficult and frustrating.’
Another question flummoxing Hanlon is how cephalopods change their camouflage so quickly – in a matter of milliseconds. ‘There has got to be a neurobiological shortcut. It’s very exciting finding out how nature has honed its elegant solutions,’ he says. His detailed observational work as he probes these questions has drawn scientists from many fields towards the wonders of natural camouflage.
Layered look
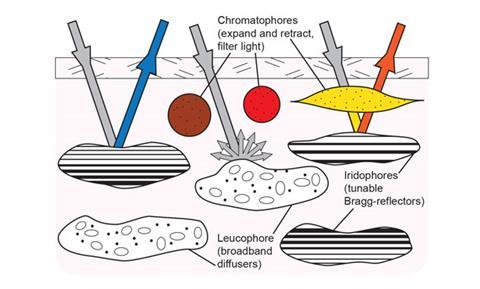
Structures contained within three layers of cephalopod skin are known to contribute to camouflage. Cells in the bottom layer, called leucophores, act as reflectors to provide an illuminating white backdrop. Next comes a layer of iridophores, comprising stacks of high refractive index (RI) films containing reflectin. The contrast between the high RI films and the low RI spaces between the films means that they act as Bragg reflectors to produce short-wavelength colours. Together, the leucophores and iridophores make blue, green and white colours. On top of these sit pigment-packed cells called chromatophores, layered yellow over red, over brown.
Cuttlefish in particular can produce a very strong white colour. Hanlon’s team has used transmission electron microscopy to study the ultrastructure of their leucophores, marvelling at how they produce ‘the whitest white’. Each cell contains as many as 12,000 spherical microparticles called leucosomes, consisting of sulfated glycoproteins and reflectin. The soft spheres may provide bio-inspiration for ways to achieve efficient light scattering in materials science and optical engineering, says Hanlon.
Each of the chromatophore’s pigment sacs contains hundreds of thousands of colour granules. The sacs expand when pulled by muscles in the skin, which are controlled by nerves originating in the brain, to give rapid colour changes. Light can interact with one, two or three of the chromatophore colours, depending on which sacs are stretched. The chromatophores act as optical filters, so that shorter wavelengths of light are absorbed by yellow pigments while longer wavelengths are absorbed by the red and brown, explains?Hanlon.
Chromatophores are so much more than simple colour filters, however, as revealed during a collaborative project between Hanlon’s team and scientists at Harvard University, US. Using tandem mass spectrometry and micro photoluminescence spectroscopy, they discovered that pigment granules taken from cuttlefish skin fluoresce at a wavelength of 650–720nm.3 This correlates with that given by two families of reflective proteins: reflectins and crystallins. Last year, for the first time, they found significant amounts of reflectin in chromatophores. This suggests that the pigment granules are more complex than the homogeneous clusters of pigments they were first thought to be.
Reflections on reflectin
Reflectin holds fascination for many researchers, not least because it is found in all three of the cephalopod camouflage layers. The proteins consist of up to six repeating subdomains and can survive high temperatures and processing.

In 2007, at the Wright Patterson Air Force Base, US, Rajesh Naik’s team was the first to characterise reflectin from a non-cephalopod source, produced using genetically engineered Escherichia coli.4 ‘We are beginning to uncover some very unusual optical properties [of reflectin] that we still cannot explain,’ says Naik. It all boils down to unusual combinations of amino acid sequences in this family of proteins, he says.
Naik’s team has also revealed that these proteins have unanticipated self-assembling properties. It was Naik’s work that brought reflectin to Gorodetsky’s attention, and he was highly excited by this particular discovery. ‘You can tune conditions so that reflectin aggregates in different ways, which maybe emphasises just how diverse its biological function is,’ he says. Naik’s team has gone on to show that reflectin can be processed into thin films, photonic grating structures and fibres, making it very attractive to materials scientists seeking technologies with potential photonic applications, such as optical sensors.

Yet the protein is horribly difficult to study, not least because of its insoluble nature. The fact that it essentially behaves as a disordered protein until it aggregates into nanoparticles and thin films makes it difficult to study using standard x-ray diffraction, says Gorodetsky. It requires some specialised techniques based on small angle scattering, he adds. ‘One has to understand the structure,’ says Naik. ‘People are having a hard time. Some are still working on trying to figure out ways to solubilise it, while others are looking for different approaches by which they can get to the secondary structure.’
Naik is waiting until a deeper understanding of reflectin’s structure emerges before he considers using it to develop materials with practical applications. He also needs to ensure that any tools he makes can be mass-produced. ‘At the end of the day, we have to be able to manufacture. Really exotic materials don’t translate into viable products,’ he says.
The only current way to produce reflectin – other than harvesting it from cephalopods – is to genetically engineer plants or bacteria to make it, which currently limits production volumes to lab use. However, Naik is hopeful for the future. Newer recombinant expression systems, where the host metabolic pathways can be tuned for recombinant protein production, can lead to increase in reflectin production volumes, he says.
Indeed, one of the first challenges that Gorodetsky faced was how to get E. coli bacteria to make large enough amounts of the protein for his studies. The final technique works well, he says, and he has been ‘more than happy’ to send genes and protocols to any researcher interested in it. ‘I would love more people to work on this protein, so I’m very excited to share the stuff that we have found,’ he says.
Gorodetsky agrees that gaining a deeper understanding of reflectin’s structure is crucial to its future use in technology. ‘You can make educated guesses and inferences, but to gain full understanding you need to be able to establish a relationship between structure and function,’ he says. For example, Gorodetsky suspects – but has yet to prove – that reflectin films transport protons so well because they are segregated into distinct hydrophobic regions and proton-conducting hydrophilic water channels.
He is working on deepening the structural understanding but is also pressing on with applications. Gorodetsky is currently leading a project to develop dynamically tunable thermoregulatory clothing inspired by the structure of squid skin, particularly reflectins.5 This type of technology would allow a wearer to harness their own radiant heat production. Such local thermal management could significantly reduce the amount of energy required to heat and cool buildings, making a big dent in US energy consumption, according to calculations by the US government’s Advanced Research Projects Agency-Energy (ARPA-E), which is funding the?project.
Gorodetsky is also keen to use reflectin in bioelectronic materials and is collaborating with Francesco Tombola, an ion channel expert at Irvine’s medical school. Electrical signals play essential functions in living cells. They are at the base of neuronal communication in the brain; they drive muscle contraction and the heartbeat; and they regulate the immune response and hormonal levels in the blood. Unlike manmade electronic devices, cells use ions rather than electrons to conduct electricity. Reflectin can conduct protons, and so the idea is to use reflectin-based materials as interfaces with living cells. ‘We are making transistors that essentially speak the same language as a cell,’ says Gorodetsky. ‘It’s a very nice collaboration between someone who’s very medically oriented and somebody who’s very materials and device oriented.’
Joint effort
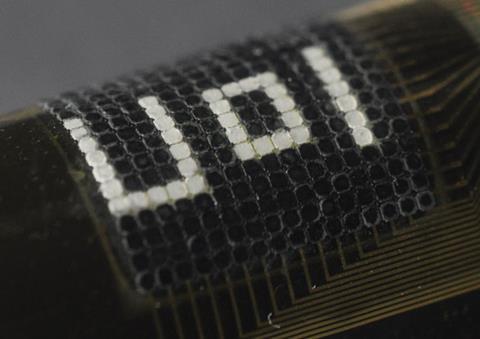
When it comes to cephalopod research, it’s all about collaboration. Hanlon is involved in a major $6 million (£4 million), four-year multidisciplinary project, funded by the US Office for Naval Research (ONR). As part of the project, Hanlon is in a team that has developed a black and white bendable camouflage device. The device consists of a thin, flexible sheet containing analogues of chromatophores, leucophores, muscles and opsins. It can be wrapped around objects and produces black and white patterns to spontaneously match the background.6
The top layer of the device contains a heat-sensitive dye encapsulated in microgranules and embedded in a polymer matrix. Beneath lies a thin layer of silver to give a bright, white background, together with an ultrathin silicon diode to provide heat and thus control the dye’s optical properties. Meanwhile, a photodiode picks up light in the surrounding environment.
‘By comparison to biology our devices are really primitive but they establish a design,’ says John Rogers, a materials scientist at the University of Illinois at Urbana-Champaign, US, and lead researcher on the project. He hopes that the black and white device will allow people to clearly visualise the direction of future research. There are ways that the device could be improved, he adds. For example, the thermal switching mechanism is not particularly attractive from a power consumption and general stability standpoint, he says.
The device team thinks that the system could be adapted to incorporate tuneable systems to mimic iridophores. Although Rogers’ work is bioinspired, he doesn’t stick rigidly to copying natural systems. ‘There might be better ways to build manmade iridophores that don’t rely on a mechanical motion [to replicate cephalopod muscle] but on [an] electrically switchable change on a molecular scale,’ he suggests.
Electronic switching technology used in some e-reader devices could prove useful, he says. The beauty of such systems is that they are very low powered. Exploiting such technologies possibly holds the key to building advanced camouflage technology, he suggests, rather than trying to reproduce an iridophore. ‘We’re not ready to deploy an active camouflage system today, but we have taken key engineering principles from biology and embodied them in a real device,’ he says.
Meanwhile, another team working as part of the ONR-funded squid skin consortium from Rice University, US, has taken inspiration from the nanoparticle nature of cephalopod chromatophores to develop a colour display system using aluminium nanorods, to improve on technology currently used in liquid crystal displays.
They arranged aluminium nanorods into well-ordered arrays to create full colour pixels. The rods act like a double slit or a prism and the colour of the pixel can be changed by altering nanorod length and spacing. These highly vibrant red, green and blue colour pixels are immediately compatible with LCD-based displays, say the researchers. Whereas dyes fade over time, the aluminium pixels will continue to give very bright colours, explains Rice’s Stephan Link.
Link has particularly relished the interdisciplinary nature of the ONR project. ‘We’ve learnt a lot,’ he says. At the heart of this collaboration sits Hanlon’s vast knowledge of cephalopods, built up over decades. Although fascinated by the minute detail, the key to Hanlon’s success is that he always has in mind the bigger picture. ‘Even when we’re looking at tiny cells, we’re still thinking about the bigger system. It’s not just cell biology,’ he says.
Emma Davies is a science writer based in Bishop’s Stortford, UK
References
- L Phan et al, Adv. Mater., 2013, 25, 5621 (DOI: 10.1002/adma.201301472)
- D Ordinario et al, Nat. Chem., 2014, 6, 596 (DOI: 10.1038/nchem.1960)
- L F Deravi et al, J. R. Soc., Interface, 2014, 11, 20130942 (DOI: 10.1098/rsif.2013.0942)
- R M Kramer, W J Crookes-Goodson and R R Naik, Nat. Mater., 2007, 6, 533 (DOI: 10.1038/nmat1930)
- ARPA-E energy efficiency projects: http://1.usa.gov/1FveiMy
- C Yu et al, Proc. Natl. Acad. Sci. USA, 2014, 111, 12998 (DOI: 10.1073/pnas.1410494111)












No comments yet