High entropy alloys, with anywhere from five or more different metals, have unusual properties and could find use in a variety of high-tech applications. Clare Sansom reports
People have been making and using alloys for about as long as they have been writing; the eponymous Bronze Age is conventionally dated from the middle of the fourth millennium BCE, about 5500 years ago. Bronze is a mixture of copper with smaller quantities of at least one other element. The very first bronzes used the metalloid arsenic with copper but most since have used tin, sometimes with zinc or another transition metal. All bronzes are harder and more durable than copper or any other metal known to antiquity, and its invention enabled our ancestors to create more complex and useful artefacts.
An alloy is defined as any substance formed from a mixture of chemical elements, at least one component of which is a metal: alloys are of course metals and thus look and behave like them, but their physical properties and therefore their applications vary enormously. All conventional alloys have one major component, which is always a metal and is known as the base or primary metal, and one or more minor ones. They are now integral to our everyday lives. Taking a ubiquitous example, almost 150 million metric tonnes of crude steel were produced worldwide in 2023. The strength and resistance of this alloy compared to its base metal, iron, are derived from the addition of carbon: adding a few percent of chromium and perhaps nickel or molybdenum to form stainless steel greatly improves its resistance to rusting and corrosion.
A typical alloy is made by melting the base metal, known as the solvent, mixing in the other molten components (the solute or solutes), casting the resulting molten alloy and allowing it to cool. In some cases, the alloy will form a single homogenous phase, with the same composition of elements and the same form throughout. Such a homogeneous mixture can be described as a solid solution. Often, however, the resulting structure will be more heterogeneous, forming crystals with different microstructures and elemental proportions. It is harder and more expensive to modify the properties of these.
The initial idea
The theoretical idea of making complex alloys from many different elements was being tossed around in metallurgical circles in the 1970s and 80s but it took a decade or so for this idea to turn into a practical reality. Jien-Wei Yeh of the National Tsing Hua University in Taiwan made the breakthrough, apparently while driving along a country road to Taipei. ‘I asked myself “Why not mix together many different kinds of elements to increase the confusion and complexity [of the alloy] and to enhance the chaos?”’ he told Materials Research Society Bulletin in 2016. Many multi-element alloys have now been developed and, perhaps counterintuitively, they are often able to form single, stable, homogeneous phases.
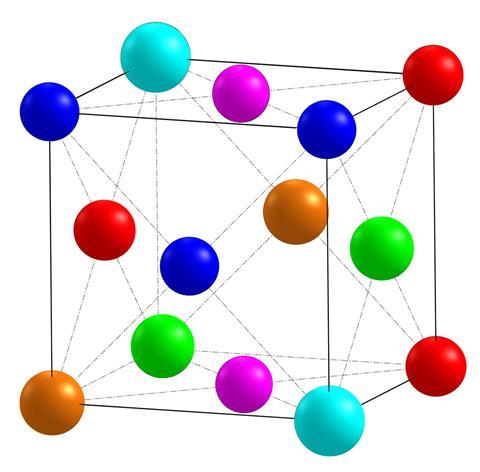
The alloys that were first envisaged by Yeh are composed of equal or near equal amounts of five or more (generally) metallic elements and are known as high entropy alloys or HEAs. The name arises because of the large increase in entropy – more specifically, in configurational entropy, or the number of different ways that the atoms can pack together – that occurs when this type of alloy is formed from its components. This increase in entropy will lower the free energy of the alloy and stabilise it to form a single-phase solid solution and, following the Gibbs free energy equation, this is more important at higher temperatures. This means that solid solution phases with simple structures can form more readily in HEAs than might be expected in materials with such complex compositions. Although five elements are typical (and some researchers class four-element alloys as HEAs), much more complex combinations are possible. One group in China has produced HEA nanoparticles composed of equal proportions of no less than 17 disparate metals, each with melting points ranging from 303–3683K and atomic numbers from 12 to 78.
Yeh has described four core effects in HEAs that derive from their fundamental atomic structure and that can be exploited to create alloys with a wide range of useful mechanical properties. These are the high-entropy effect, sluggish diffusion, lattice distortion and the cocktail effect. The high-entropy effect is simply the basic statement that the high configurational entropy of these alloys facilitates the formation of simple solid solution phases. Diffusion here refers to the rate at which atoms jump into vacant neighbouring lattice sites, and this is slowed in HEAs because these sites have different neighbouring atoms (and therefore differ in size and valence). These size differences also lead to distortions in the shape of the lattice, causing additional strain. The cocktail effect refers to the importance of the interactions between component elements – as well as the properties of individual ones – in determining the properties of the alloy.
This use of lattice disorder and high configurational entropy to stabilise materials was soon applied to other materials, including the high-entropy metal chalcogenides (HEMCs), which are ionic compounds formed from multiple metal cations and group 6 anions: mainly sulfur, selenium and tellurium. Compounds that include the lightest group 6 element, oxygen, are generally treated as a class on their own. ‘The HEMC lattice is a highly distorted version of their binary chalcogenide cousins,’ explains David Lewis of the University of Manchester, UK. ‘Adding further metals into the lattice increases this disorder and distortion and can produce properties that are ideal for electrocatalysis.’
Yeh names Brian Cantor, then of the University of Oxford, as another pioneer in the HEA field. Cantor began researching this area in the 1970s but, originally unaware of Yeh’s work, did not publish until 2004. And he is the only researcher to have an HEA named after him. The Cantor alloy, which he developed, is made from equiatomic quantities of five metals: chromium, manganese, iron, cobalt and nickel (CrMnFeCoNi). This forms a single-phase solid solution with a face-centred cubic structure, which is common in HEAs. It is extremely ductile, particularly at low temperatures, and it increases in strength as its temperature is lowered down to about 196K (–77°C). Its physical properties can be altered by adding further elements in small quantities or by slightly altering its elemental composition.
Characterising the crowd
The direct relationship between the atomic structure of an HEA and its properties (and therefore its potential applications) is also seen in many other areas of chemistry. There is one key difference, however. In most sub-disciplines, atomic-level structure is all-important. In contrast, as Lewis Owen, a research fellow at the University of Sheffield, UK, explains, ‘Metallurgists need to understand the structure of HEAs at all length scales: at the atomic level (in its crystal structure) but also at micro- meso- and macro- (centimetre and above) scales.’ Microstructure refers to structure on the scale of around a micrometre and describes how a material’s crystals fit together to form plates, needles or cubes..
The techniques used for studying HEAs at the micro- level and below, however, are very similar to those used elsewhere in science: x-ray diffraction, neutron diffraction and scanning electron microscopy, and the methods used to determine the atomic structure of an alloy are are similar in all these disciplines. ‘One difference with an HEA – or any other metallic sample – might be in the type of experiment we carry out,’ says Owen. ‘While with protein crystals you have limited time to do your experiment before the crystal degrades, here you can probe the materials in operando – that is, under operational conditions – so you can find out what happens to the structure of your HEA at a temperature of over 1000°C, or under very high pressure, if that is relevant to its application’.
The design of an HEA for a particular application is a complex matter. There are a large number of elements to choose from, and any set of five or more can be combined in very many ways. The process of alloy design often starts with modelling the thermodynamics and physical properties of the potential alloy through the calculation of phase diagrams. The Calphad software suite – the name is derived from calculation of phase diagrams – is based on methods that were originally developed in the 1970s but are still widely used. It takes an empirical approach and is used in combination with a range of regularly updated databases, each covering a particular class of material. The HEA database includes data for about 540 specific phases from combinations of 26 different elements, all but two of which are metals: the non-metals included are carbon and nitrogen. ‘The design process normally involves predicting the properties of an HEA from a given combination of elements, making the alloy, comparing its properties against the prediction, tweaking the composition and cycling through until the material has the properties we are looking for,’ adds Owen.
The route to applications
High entropy alloys can be used for a wide range of applications, but, as they are complex and expensive to manufacture, these are generally restricted to those that take place in extreme environments. ‘If there is a cheap steel available that will do a particular job – and there often is – why wouldn’t you use it?’ says Ed Pickering of the University of Manchester, UK. ‘We need to use HEAs and other exotic materials only for those applications where only they will do.’
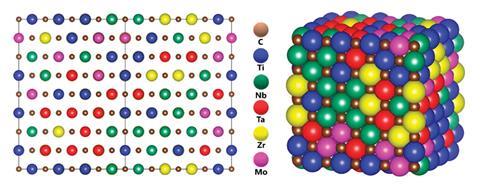
Those applications for which only an HEA will do include sorption, or the storage of hydrogen and, potentially, other gases in the gaps in the distorted lattice; making parts of jet engines that have to operate under high temperatures and pressures, and in reactors for nuclear fission and potentially fusion. As the climate crisis intensifies, nuclear energy is now increasingly seen as part of a low-carbon energy mix and improving the efficiency and, above all, safety of nuclear reactors is becoming a key research priority.
Nuclear fusion power, in which energy is produced by the fusion of two light nuclei, may be considered the pinnacle of high-tech low-carbon energy generation, using fuel components that are readily available. Yet, despite 80 years of research, no successful prototype reactor has yet been produced.
The many challenges that remain include the selection and manufacture of suitable materials for the inner walls of the reactor, which will be in contact with radioactive plasma. The requirements for these are stringent and no conventional material meets them fully, but it might be possible to design an HEA with suitable properties. It will need to be exceptionally strong and tough, able to withstand temperatures of up to about 1000°C and the damage from a neutron flux of up to about 1015 neutrons per cm2 per second. Radiation damage is measured at the atomic level in displacements per atom (DPA); the damage from this flux is estimated at about 10 DPA per year, which means that each atom will be displaced from its lattice point about 10 times a year.
Furthermore, these neutrons have a wide range of energies, and high- and low-energy neutrons affect atomic structures in different ways. ‘A low-energy neutron can be captured by an atomic nucleus, releasing a photon; a high-energy one can knock a proton or neutron out of the same nucleus,’ explains Mark Gilbert, head of the fusion interface programme at the UK Atomic Energy Authority. ‘The net result in each case is a transmutation [of one element into another]: a collision with a low-energy neutron will move an atom one place to the right in the periodic table, and a collision with a high-energy one will move it one place to the left.’ As an illustration, the transition metal tungsten (Z = 74) can be transmuted into rhenium (Z = 75) by low-energy neutrons or tantalum (Z = 73) by high-energy ones.
This limits the choice of metals for the construction of those parts of a fusion reactor that will be vulnerable to neutron flux to those that cannot be transmuted, either directly or indirectly, into radioactive isotopes with long half-lives. The criterion that is generally used is the length of time before the radioactive material generated can be disposed of as low-level waste. Tungsten’s transmutations cannot produce isotopes that remain radioactive for too long, but those of many other metals can, including nickel, which is often used as a component of HEAs. ‘Once you remove all the elements that are transmuted into highly radioactive isotopes, there is only a small fraction of the periodic table left to make into combinations of five or more elements to form an HEA,’ adds Gilbert.
The elements left do still provide plenty of possible combinations, however, and the palette will expand for any that are to be included in small quantities. And there is still sufficient leeway to optimise the other properties that are required, including ductility and strength, and, perhaps, the cost of the components. A fusion reactor built on an operational scale using the materials that are currently being considered would cost billions, but, unless the elements chosen are intrinsically very rare, economies of scale can kick in if the technology matures.
Whether or not fusion power ever becomes a mature technology, there are many others that will find further applications for HEAs and the other exotic high entropy materials.
Clare Sansom is a science writer based in Cambridge, UK
This article was updated on 21 March 2024 to clarify some aspects of nuclear fusion, thanks to suggestions from Mark Foreman of Chalmers University of Technology in Göteborg, Sweden.

















No comments yet