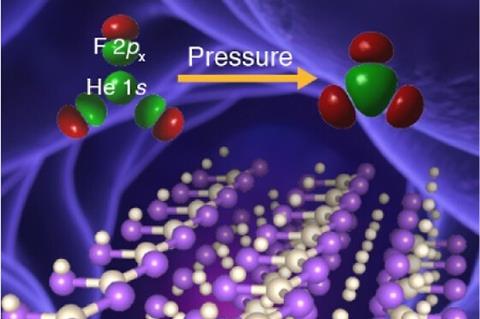
A team of researchers in the US and China has calculated that extreme pressure could theoretically allow stable covalent helium–fluorine bonds to form, challenging the idea that helium is a chemically inert element.
Noble gases get their name due to their inherent reluctance to react. Their complete electron configurations lead them to have exceptionally high ionisation energies, resulting in an unwillingness to form compounds. Despite this, there have been numerous examples of heavier noble gas compounds forming under non-ambient conditions, notably a range of xenon fluorides. Helium equivalents, however, are much rarer, with such compounds often being unstable or existing only briefly as transition states.
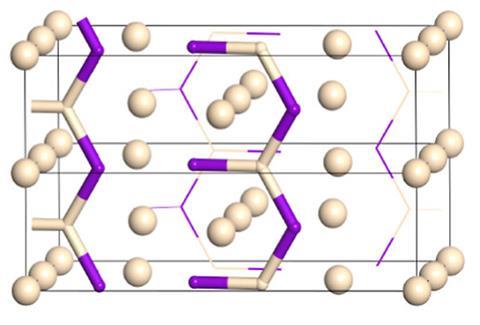
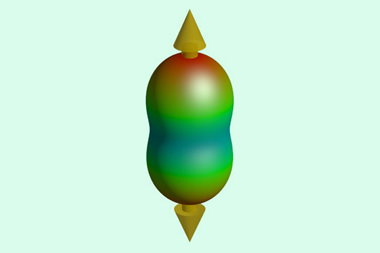
Now, using an algorithmic search that varies the chemical composition and pressure, scientists have identified a compound where helium actively participates in chemical bonding. At pressures in the tera-pascal range – about 10 times the pressure found at the centre of the Earth – the energetically stable compound He3F2 forms. This molecule consists of HeF2 chains and interstitial helium atoms. Within each chain, helium forms polar covalent bonds to three fluorine atoms, with helium donating electron charge. Molecular orbital calculations of each HeF3 cluster reveal that extreme pressure allows the helium 1s and fluorine 2p orbitals to form bonds.
The emergence of helium–fluorine bonding further challenges the idea that noble gases are unreactive. The researchers say that helium-bearing interiors of giant planets may contain similar compounds. Such extreme pressures needed to synthesise these materials may be achievable in the lab, but only at a few select research facilities.
References
J Hou et al, J. Am. Chem. Soc., 2025, DOI: 10.1021/jacs.5c06707













No comments yet