Chad Mirkin has spun out his nanotechnology research into several companies

Chad Mirkin has been the driving force behind four spin-out nanotechnology companies, and has more than 850 patents to his name. Yet he describes himself as something of an accidental chemist. ‘I had three brothers who were in science,’ he says. ‘One was a physicist, one a biologist, and one a geologist. That left chemistry. We were a very competitive family!’
At the outset, he anticipated his chemistry studies at Dickinson College in Carlisle, Pennsylvania, would lead to medical school, but around his third year, he realised that was about the last thing he wanted to do. ‘I did a hospital rotation and decided it really wasn’t for me,’ he says. ‘I had a bit of a crisis and looked into other possibilities, including a research experience course for undergraduates at Pennsylvania State University. I caught the chemistry bug from a really inspiring scientist there, Greg Geoffroy.’ Mirkin even published three papers as an undergraduate, giving him the confidence in science to go to graduate school, working in Geoffroy’s labs.
In 1989, Mirkin finished his PhD, on synthetic organometallic chemistry, in a little under three years – almost unheard of in the US system – and Geoffroy suggested he should go to the Massachusetts Institute of Technology (MIT) for a postdoc with Mark Wrighton. This widened his chemistry interests from pure synthetic to surface chemistry, electrochemistry and materials chemistry. He also managed to merge some of the concepts from his graduate work into his projects with Wrighton to create a new class of electrochemical sensors for carbon monoxide.
In 1991, the visibility that work gave him helped him land a job at Northwestern University in Illinois, US, initially as assistant professor. ‘At that time, there was a lot of excitement about scanning probe microscopes, and Northwestern had some of the early commercial ones,’ he says. ‘I trained myself to do scanning probe microscopy along with my group, and we had soon built one of the largest scanning probe microscope capabilities in the world, inventing a technique called dip pen nanolithography along the way.’
Nanotech nous
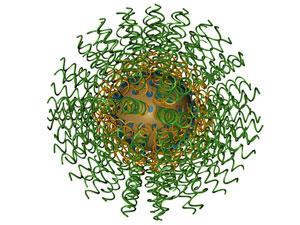
In parallel, Mirkin developed an interest in nanoparticle chemistry, introducing what he termed a programmable atom equivalent, or spherical nucleic acid. A nanoparticle of some form is at the particle’s core, acting as a scaffold around which DNA oligonucleotides are arranged. ‘The idea was to think of a particle as an atom, functionalise it with molecules with a high information content like DNA, and then use these programmable atom equivalents as building blocks to assemble materials from the bottom up, using DNA to make them come together,’ he says. ‘In the process, we discovered the particles have some spectacular properties that go beyond materials science, with potential impact in many areas of biology and medicine, and their fundamental properties make them very attractive as probes in biological detection.’
In spite of these properties, he says, the commercial possibilities were not immediately obvious. ‘There are two approaches to invention and translation of work. One is to consider a problem as one of engineering, and solve it by applying the right engineering. All the fundamental science has already been done – it’s a case of putting the right things together,’ he says. ‘The other approach involves a desire to do and make new things, look at their fundamental properties, and ask how they might be used to develop new technologies.’
Mirkin was trying to create a new way of making materials via programmable assembly, not looking for biological probes. But he quickly realised that when these particles are joined together by DNA linkers, their properties change. ‘The dispersed particles have one set of properties that are, for example, red in colour, and when assembled they have a different set of properties, blue in colour,’ he explains. ‘The idea that this could be used to develop a new colorimetric detection system was the birth of Nanosphere, which is now a public company.’
Mirkin’s curiosity was piqued, and he started to wonder what else might be done with the technology. He realised the particles might also be used as amplifiers, as they will catalyse reactions to generate large signals, despite their very low concentrations, increasing the sensitivity of detection. Add them to microarray chips, and the result could be – and was – commercialised. ‘The chips are, essentially, a spotted array of DNA on a surface,’ Mirkin explains. ‘The sample contains DNA targets in solution, the chip is exposed to that sample, and the targets go to the correct point on the chip. It is then developed with a nanoparticle probe that can “sandwich” the captured particles.’
The gold-centred nanoparticle probe, or spherical nucleic acid, gives a red colour. The signal is weak, but if silver photographic developing solution is flowed over the chip, any point where gold is present becomes stained with silver, increasing the signal by a factor of 100,000 in less than five minutes. The result is the most sensitive non-PCR detection system currently available. There is a problem, however, as the DNA binding is not perfectly selective: the strands in the sample bond not only to the perfectly complementary spots, but can also bond to those with a majority of complementarity. This would give a false positive. ‘These nanoparticle probes have very sharp melting transitions, and on heating they dissociate over a very narrow temperature range,’ Mirkin says. ‘Thus a temperature can be picked at which only the perfectly complementary ones are bonded – at a broader temperature range, there would be some non-complementary ones, too.’
Sense and selectivity
Assays using this technology are both extraordinarily sensitive and very selective, he says. ‘When you’re trying to detect many targets at once, that’s critical, as otherwise there is a real problem deconvoluting the data. At Nanosphere, the technology has been streamlined, and it has nine clearances for panel assays from the US Food and Drug Administration.’ Identifying sepsis is an important application, which Mirkin claims no-one else can do as rapidly as his test. ‘It can identify bloodstream infections within a couple of hours, while the current tests take three days. This is a life or death situation – every hour it’s not diagnosed, mortality increases. So the patient is given an expensive battery of antibiotics, which they may not even need. This technology allows all the potential bacteria to be identified quickly, saving time, money and lives. It’s a real game-changer.’
There are plenty of other diagnostic possibilities, such as genetic tests to identify people with a predisposition to develop blood clots, or the one-in-three people who cannot metabolise the common blood-thinning drug warfarin. It can also be applied to other infectious diseases, including all the different forms of flu.

Nanosphere was spun out in 1998, taking on venture capital investment in 2000 before going public in 2007. Based in Northbrook in Illinois, it now employs about 160 people. A second spin-out, NanoInk, set up in 2002 to exploit the dip pen technology, didn’t make it, although its lithography systems continue to be commercialised and used as research tools around the world. Two further, more recent, spin-outs, Aurasense and Aurasense Therapeutics, are still going strong. Thus far, Mirkin’s inventions have attracted more than $700?million of venture capital and related investment, and more than 400 commercialised products.
Detection and therapy
Aurasense was set up in 2009 with angel investment backing, and has commercialised diagnostic probes to detect RNA. These are sold by Merck Millipore under the trade name SmartFlare. There have been more than 300 SmartFlares devices sold so far, and by the end of the year Mirkin expects the number will top 1000. The probes use particles very similar to those used in the Nanosphere system – gold particles with a shell of DNA – but hybridised to that shell is a short oligonucleotide bearing a fluorophore, creating particles Mirkin terms nanoflares. Gold quenches the fluorescence so they are initially spectroscopically silent. However, unlike linear DNA, these spherical nucleic acids engage scavenger receptors and are taken into cells by endocytosis. This makes it possible for particles bearing large amounts of DNA or RNA to enter cells without the need for potentially toxic polymeric co-carriers.
‘They have no significant effect on the cells,’ Mirkin says. ‘But they can be designed to recognise mRNA [messenger RNA] inside cells, and on binding release the fluorophores, selectively lighting up cells that contain the mRNA they are designed to recognise.’ Combined with flow cytometry, they can be used to pinpoint cancer, for example, selectively lighting up in the presence of a gene that is overexpressed in cancerous cells. Stem cells can be detected in a similar way.
They can also be used to look at how drugs change a cell. ‘Which are the best drug molecule candidates in a library to target a particular gene? By taking many reactors with the same type of cell and feeding them the same type of nanoflare but different drug molecules, it can show which triggers an under- or over-expression of a gene,’ Mirkin says. ‘This is revolutionising many aspects of cell biology because, for the first time, gene expression can be measured at the single cell level in live cells.’
These gold-encapsulating spherical nucleic acid particles also have potential as therapeutic agents, leading to the most recent of Mirkin’s spin-out companies, Aurasense Therapeutics, set up in 2011 and financed by the venture capital arm of pharma company Abbott (now AbbVie). It takes advantage of the particles’ ability to enter cells – they can even be put on the skin, where they will penetrate the stratum corneum. ‘For example, we can carry out knockdown experiments in the skin of animals, where we can change what the keratinocytes [skin cells] express,’ he says. ‘So you can begin to target diseases like melanoma or psoriasis, facilitate wound healing, and it may have anti-scarring capabilities.’
Oncology is another potential application. When systemically injected, the particles are distributed in all manner of tissues that normal gene regulation agents will not enter, and can even cross the blood–brain barrier. ‘All the drugs that are being developed in the gene regulation space at the moment are for diseases of the liver, as that’s where they tend to end up when they are administered,’ Mirkin says. ‘But the spherical nucleic acid particles go everywhere in the body – that’s either good or bad, depending on what you want to do with them! But for a brain cancer like glioblastoma, the drug has to get across the blood–brain barrier. We are seeing great examples where the particles get into the brain, are localised in tumours, and target genes within the tumour cells, causing the cancer cells to die selectively. It’s very exciting – it could open up the ability to go after many forms of cancer with a genetic basis, and also neurodegenerative diseases such as Alzheimer’s and Parkinson’s.’
Mirkin believes the company has many benefits to bring to the market. ‘This technology has the chance to completely transform gene regulation therapy, because it addresses some of the biggest problems that limit the field, particularly biodistribution,’ he says. ‘Another advantage is stability – the lifetime of these spherical nucleic acids is typically an order of magnitude greater than the same linear nucleic acid. In addition, they do not stimulate an immune response, with their stealth-like characteristics allowing relatively simple oligonucleotides to be used as therapeutics, in contrast to the modified nucleotides that are required with more conventional approaches to increase stability and decrease the activation of immune response. Any disease that has a genetic basis is a candidate for the development of an spherical nucleic acid-based therapeutic, from cancer to wound healing. These have real potential to improve the lives of patients.’
He is particularly proud of the spherical nucleic acids. ‘It’s a fundamental new form of DNA and RNA,’ Mirkin says. ‘It’s not just different in terms of structure, but also in terms of function. On a sequence-for-sequence basis, even though they are made out of the same building blocks, they’re fundamentally different in terms of where they can go, their ability to enter cells and bind with complementary nucleic acids, how they bind and how they melt. It’s a paradigm shift in the way we think about nucleic acids, and the way nucleic acids can be used to develop powerful new diagnostic and therapeutic tools.’
Mirkin is now fairly detached from the furthest advanced of the companies, Nanosphere, stepping down from the board of directors this year, but it still licenses more than 300 of his patents. He remains intimately involved with both Aurasense and Aurasense Therapeutics, which are at a much earlier stage of the commercialisation process. And he is certain there will be more companies in the future.
Sarah Houlton is a science writer based in Boston, US











No comments yet