The sustainable synthesis of pharmaceutical, agrochemical and material chemistry scaffolds is one of the most urgent problems for the chemical community. To meet this challenge, significant improvements in cross-coupling catalysis scope, selectivity and efficiency have been realised through the development of a variety of ligands.
These efforts can give the impression that the cross-coupling chemical space is now thoroughly populated and understood, yet many opportunities remain. In this article, we’ll showcase the advances in transition metal catalysis that have been driven by the research and design of sophisticated phosphine ligands, specifically looking at:
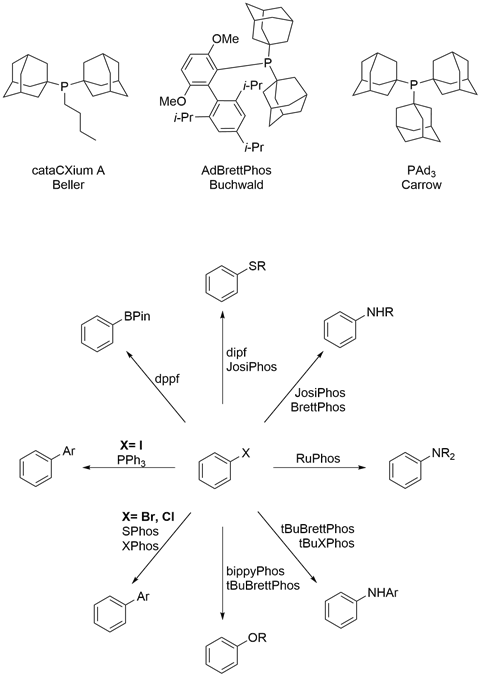
- Tris(1-adamantyl)phosphine – a bulky, electron-rich ligand, developed by Brad Carrow’s research group
- YPhos ligands – a novel class of phosphines bearing ylide substituents, developed by Viktoria Gessner’s research group
The next generation of ligands for complex coupling challenges
As the world understands more every day, sustainability is imperative for both worldwide equity and further economic growth. Chemical companies must play a dual role in addressing this challenge by advancing more sustainable solutions and improving their chemical processes to minimise their own environmental impact. This means process chemists are faced with the challenge of synthesising more complex targets in fewer steps, minimising the use of scarce resources and reducing the waste a process produces.
Since its discovery and development in the latter half of the 20th century, metal-catalysed cross-coupling has become an indispensable tool in the preparation of drug molecules, agrochemicals and electronic materials.
Enabling chemists to link aryl rings together or with other groups via carbon–carbon bonds created new possibilities for synthesis that simply could not be envisioned before. Over the course of the last 70 years, cross-coupling has grown and adapted with an array of new ligands being developed allowing it to fulfil a range of new uses in synthetic chemistry. This ability to adapt to chemists’ needs now offers a solution to contemporary sustainability challenges by helping create processes that transition the world to a greener tomorrow.
A cornerstone of modern organic synthesis
The path to harnessing the power of metal-catalysed cross-coupling starts with palladium, which remains the metal most commonly employed in cross-couplings to this day. And the key to unlocking the properties of palladium is in the ligands surrounding it. Several researchers independently discovered that sterically hindered, electron-rich phosphines play a crucial role in improving the efficiency and tolerance of the palladium catalyst.

Since this time, several new phosphine ligand scaffolds have been developed, each tuned for specific properties. Thanks to the availability of more specialised ligands, the scope of cross-coupling now encompasses carbon–heteroatom bond formation as well. Today, fertile opportunities remain in cross-coupling to push forward the wide range of fields that the chemistry is used in.
Pushing the boundaries of ligand capabilities
Though any advance in our scientific understanding could carry over into a practical solution, only a fraction of innovations in academic research are implemented in real-life, commercial applications. The progress in ligand design that has been facilitated by Umicore PMC’s academic partners are directly relevant to industrial challenges.
Brad Carrow’s group at Princeton University, US, synthesised tris(1-adamantyl)phosphine (PAd3). In subsequent studies, they found that palladium–PAd3 complexes catalyse Suzuki-Miyaura couplings with an exceptional turnover number (TON) – the number of times a catalyst can be used before it’s expended – of nearly 20,000. In practical terms, the high efficiency displayed by palladium complexes of these new phosphines allows for short reaction times, even without heating. This gives the process chemist a more flexible range of temperatures to work within, allowing for broader compatibility with sensitive functional groups or for simplifying the process by enabling a room temperature reaction. These less extreme conditions open up greener synthesis pathways for chemists.
Another example of applying advances in ligand design to industrial challenges can be found in recent work by Viktoria Gessner’s group at Ruhr University Bochum, Germany, exemplifying how an understanding of structure–activity relationships can pave the way toward more effective catalysis. Through exploration of new ligand architectures, Gessner and coworkers have discovered a class of ylide-containing phosphines they have dubbed the YPhos family. The ylide substituent imparts strong electron donating abilities to the phosphorus donor site.
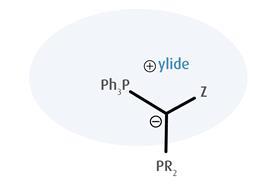
The electron richness of the YPhos ligands leads to uniquely high activity in cross-coupling reactions, especially Buchwald–Hartwig aminations. Additionally, while ylides are normally known to be very reactive species, YPhos ligands tend to be surprisingly stable when complexed with a metal owing to additional interactions between the ligand and the metal species. During development, steric demands as well as donor properties were examined.
Innovations with practical benefits
Taken together, these ligands represent a versatile solution for a number of cross-coupling applications.
The low amounts of the catalyst required, enabled by both the YPhos and PAd3 ligands, means less palladium is required. This means less metal must be removed from the product to meet regulatory guidelines, helping to reduce downstream costs. Minimal catalyst loading also minimises the impact of the precious metal catalyst on the supply chain. Less palladium needs to be managed in the loop, reducing the cost as well as the complexity involved.
The key to a more sustainable future is increased collaboration between industry and academia
Finally, in order for innovations to be practically implemented, intellectual property (IP) rights must be in order and supply secured for commercial use. Umicore has negotiated IP with respective universities and can supply catalysts bearing YPhos and PAd3 ligands. As a result, any additional cost from licence agreements is included in the price of the catalyst, and no further negotiation is needed, removing barriers for its use.
In recognition of the potential of these technologies, Umicore has leveraged precious metals chemistry expertise and industry understanding to ensure a strong supply of these innovative ligands to the market. Technologies like these demonstrate how the boundaries of innovation are always being pushed. Despite being over 70 years old, the field of cross-coupling catalysis continues to flourish and adapt to the needs of tomorrow.
Collaboration is key
The sustainable synthesis of pharmaceutical, agrochemical and material chemistry scaffolds will define the next era of the chemical industry. As these discoveries show, cross-coupling catalyst design is fertile ground for finding technologies to meet this need. However, a major part of the discovery is effective collaboration between industry and academia. As part of our collaboration with Gessner’s group on YPhos ligands, for example, Umicore provided a perspective informed by our ongoing relationships with process chemists at dozens of companies to help focus the research on challenges we knew our clients faced. Doing so allowed for more market-relevant discoveries to be deeply explored. Likewise, academia can often provide innovative ideas and resources that businesses can’t find elsewhere.
The key to a better, more sustainable future is increased collaboration between industry and academia and it’s up to businesses to embrace that future. By nurturing the relationship between the two, both sides can unlock their true potential. As a technology leader in the field of catalysis, Umicore is positioned to help bridge the gap from academic discovery to a practical, scalable solution. Together, we can co-create for success.
Bio: Philip Wheeler, business development manager at Umicore Precious Metals Chemistry (PMC)

Through his expertise, experience and data-driven methodology, Philip drives Umicore’s collaborative approach to inspiring excellence and innovation at the intersection of science and business for our partners.
In his career, Philip has worked on both the lab bench and office desk. And today, in his role at Umicore, he specialises in advancing the academic and commercial applications of innovative homogeneous catalysts.













No comments yet