The stability of the electrochemical reduction of carbon dioxide can be dramatically improved simply by adding a small amount of acid. This addresses a key stumbling block in the commercialisation of the reaction by dissolving the salts that block the reactor and rapidly lead to failure.
The electrochemical reduction of carbon dioxide is potentially a huge advance in green chemistry as it uses electricity to reduce carbon dioxide into important platform chemicals such as carbon monoxide and hydrocarbons. This could transform carbon-positive syntheses into carbon-negative ones.
An especially promising design for the electrolyser is the membrane electrode assembly. This incorporates two electrodes pressed against an anion exchange membrane. The cathode is a gas diffusion electrode continuously fed with carbon dioxide and water vapour. At the anode, oxygen evolution from water releases electrons that travel through the external circuit. However, these electrons can reduce either carbon dioxide or hydrogen, and owing to the stability of carbon dioxide, in the absence of other influences hydrogen reduction is strongly favoured.
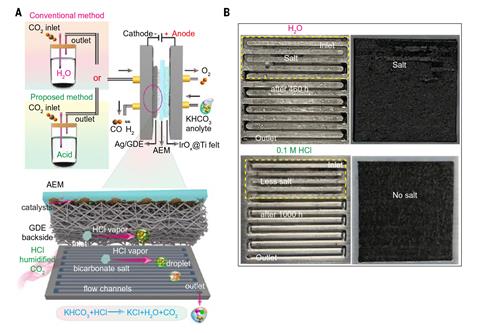
Researchers, therefore, usually add alkali metal salts to the anode electrolyte. ‘What people have shown in the literature – and this is kind of an ongoing debate on the exact mechanism – is that the cations will have some electrostatic interactions … that would do this initial activation step [of carbon dioxide],’ explains electrochemist Ahmad Elgazzar at Rice University in Texas. Unfortunately, at neutral pH the cations can also react with the carbon dioxide to form bicarbonates or carbonates, which have limited solubility in water and can clog the gas diffusion electrode, leading to pressure buildup and failure. Various solutions to this problem, such as membrane modification and pulsed electrolysis, have been investigated, but none have proved totally satisfactory.
In the new work, conducted with potassium salts, Elgazzar and colleagues in Haotian Wang’s group at Rice University in Texas simply added hydrochloric acid to the water vapour. They found that, whereas membrane electrode assemblies run using pure water showed rapid degradation in activity and failed after less than 500 hours, assemblies supplied with a feed containing concentrations as low as 0.05M acid could work stably for up to 4500 hours. Moreover, although disassembly of the acid-humidified assemblies showed that some salt-deposition had occurred, the researchers do not believe that this was the cause of their eventual failure as they had not observed a sudden pressure drop in gas flow across the cell before it stopped working.

The researchers went on to demonstrate that the idea worked equally well with other, less corrosive acids. ‘Potassium formate, potassium acetate – these salts all have much higher solubility than potassium bicarbonate,’ says team member Shaoyun Hao. A technoeconomic analysis suggested that, owing to the tiny quantities of acid consumed, there was a clear cost benefit to implementing the scheme.
Chemical engineer Cao Thang Dinh at Queen’s University, Canada, is impressed. ‘Previously, we’ve tried to avoid this problem by minimising it,’ he says. ‘People have run [the reaction] at very low electrolyte concentration so they can avoid the salt formation. The downside of that is that when you run at very low salt concentrations you have a high cell resistance, so the overall energy conversion is low… Now they run at high salt concentrations but have similar or better stability.’ He concludes: ‘I’m surprised many people didn’t try this before because it’s so simple and yet it works. I think it will have a huge impact on practical applications.’
References
S Hao et al, Science, 2025, DOI: 10.1126/science.adr3834













No comments yet