Orbital-resolved contributions provide a fresh perspective on hydrogen bonds with covalent characteristics
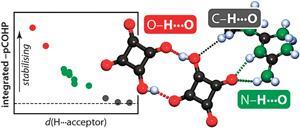
Covalency, a term describing bonding by sharing electrons, divides opinion when mentioned alongside hydrogen bonding. Worried that the concept of hydrogen bonding has been getting fuzzier over time, scientists in Germany have sought a fresh look at the very nature of these bonds, and how much covalency they involve.
Richard Dronskowski and colleagues at RWTH Aachen University collected evidence from hydrogen-bonded molecular crystals to elucidate how these crystals are held together and compare the covalency of long and short hydrogen bonds. To do this, they performed state-of-the-art periodic ab initio calculations, which can dissect the electronic energy into orbital-resolved contributions and measure the degree of covalency in all hydrogen bonds for comparison with structural details. ‘This provides a very different perspective from what is usually used in molecular crystallography – ie charge density analysis – and it also differs from standard gas-phase molecular calculations. And the results fit,’ explains Dronskowski.
Hydrogen bonds are cornerstone interactions in chemistry and biology. However, the ‘hydrogen bond’ label comprises a complicated melange of covalent, ionic and dispersive interactions, very different in nature and strength and often the subject of controversy.
Slawomir Grabowski, a hydrogen bond expert from the University of the Basque Country, Spain, says this imaginative orbital-based approach will help deepen our understanding of these interactions. He says the role of strong and very strong hydrogen bonds is regularly the subject of special attention, because these interactions are central to the proton transfer reactions in numerous biochemical processes. ‘Strong hydrogen bonds are often characterised by properties typical for covalent bonds.’
But how much covalency is involved, exactly? ‘Our results do not suggest a strict border between covalent and non-covalent hydrogen bonds but show a gradual transition, corroborating nicely with traditional wisdom and previous results, but on the grounds of very different techniques,’ stresses Dronskowski. ‘The nature of the strongest hydrogen bonds indeed compares to those bonds that connect atoms in everyday molecules. On the other hand, not all contacts between hydrogen donors and acceptors contribute pairwise stabilisation.’
Indeed, the study flipped conventional assumptions surrounding intramolecular H…O contacts in one particular crystal structure to classify it as nonbonding. And this is where Dronskowski’s tool, the crystal orbital hamilton population (COHP) technique, may prove most useful – for assessing contributions from short intramolecular contacts. ‘For anyone working on molecular crystal structures,’ he warns, ‘be careful about drawing far-reaching conclusions based on geometry alone.’
References
V L Deringer, U Englert and R Dronskowski, Chem. Commun., 2014, 50, 11547 (DOI: 10.1039/c4cc04716h)










No comments yet