Catalyst offers cheaper route to disinfect water
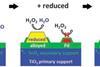
Nanoparticles can be used to produce small amounts of hydrogen peroxide with less precious metals in straightforward process

Nanoparticles can be used to produce small amounts of hydrogen peroxide with less precious metals in straightforward process