The mystery of the ‘ouzo effect’ – where water added to the clear alcoholic spirit suddenly turns the entire drink milky white – has been explored in greater depth by chemists using a specialised electron microscope. The team behind the work hopes that it could lead to better emulsions in instances like paints, drugs and plastics.
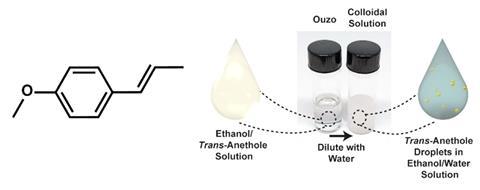
Scientists have known for years that the ouzo effect – it’s also seen in similar spirits like pastis and sambuca – is caused by the drink’s three transparent components: water, ethanol and the anise extract anethole forming a light-scattering emulsion instead of mixing completely. But the resulting emulsion is not well understood and the fact that it’s highly stable and doesn’t need a surfactant to stabilise the interface of the components makes the ouzo effect even more curious. ‘It’s an inherent process to the assembly of the molecules,’ says study author Nathan Gianneschi, a professor of chemistry at Northwestern University, US.

Gianneschi and his colleagues investigated the ouzo effect1 by examining droplets of the resulting emulsion with a liquid phase transmission electron microscope (LPTEM)2, which can image hermitically-sealed slides containing samples of liquid. They found that the droplets of ethanol and anethole – a hydrophobic oil – didn’t grow larger than a few micrometres across, but instead formed a more pronounced ‘bubble-like’ structure, with a dark ring on the outside and a paler interior.
The researchers think each droplet has high concentrations of anethole at the edges and ethanol at the centre, and that this internal structure gives the emulsion its high stability, which can last for days. ‘The droplets don’t continue to grow and become destabilised,’ Gianneschi explains.
It’s the first time that an LPTEM has been used to examine the ouzo effect, and it shows the potential of the instrument to minutely image liquids and other substances that can’t be examined otherwise. ‘In the last eight years, there’s been a huge flurry of activity and utilising the technique to image soft matter,’ Gianneschi says. ‘It’s really been one of those times when the technology enables science.’
He hopes that a better understanding of the ouzo effect could lead to highly stable emulsions for other applications, such as making paints, glues and many plastics. Knowing more about emulsions is also valuable to the oil industry, and it could help to address environmental damage caused by oil spills, he says.
Physical chemist Hervé This, the director of the International Centre for Molecular Gastronomy at AgroParisTech-INRAE, has also investigated the ouzo effect, but in Pernod. He says some earlier studies reached some of the same conclusions. But ‘I like the experimental setting, and I like how they have validated the results using this method’, he says.
References
1 MA Vratsanos et al, ACS Cent. Sci., 2023, DOI: 10.1021/acscentsci.2c01194
2 H Wu et al, Adv. Mater., 2020, 32, 2001582 (DOI: 10.1002/adma.202001582)












No comments yet