The news of a brand new class of antibiotic and a promising clinical candidate, zosurabalpin, against the priority pathogen, Acinetobacter baumannii has been met with great excitement around the world.1,2 Not only has it been a long time since a new class of antibiotic was discovered, but A. baumannii is a pathogen for which the number of available treatment options is rapidly dwindling.
What is the new antibiotic class and how does it work?
The new class of antibiotic is a macrocyclic peptide, which represents a structurally distinct compound class with a different mode of action to any antibiotic currently in use. The novel antibiotic class was discovered by screening a library of 45,000 macrocyclic peptides, produced by Tranzyme Pharma using solid-phase parallel synthesis, against a range of human pathogens.
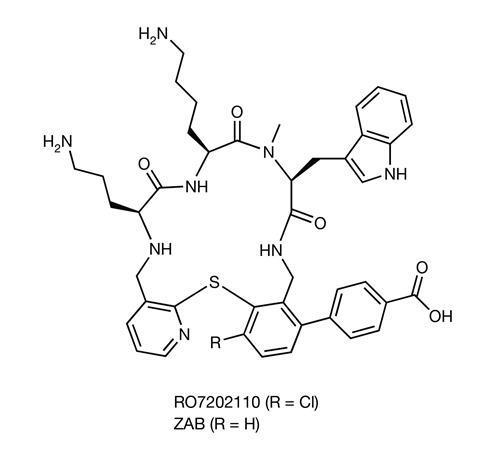
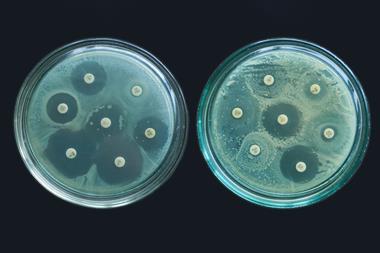
The new class of antibiotic has been found to have potent activity against carbapenem-resistant A. baumannii (Crab) and works by blocking the transport of lipopolysaccharide (LPS) – an important outer membrane component of gram-negative bacteria – from the inner membrane to its destination on the outer membrane. These macrocyclic peptides do this by inhibiting a complex of proteins located between the bacterium’s inner and outer membrane layer. Inhibition of LPS transport causes these bacterial molecules to build up to toxic levels inside the cell, eventually killing the bacterium.
‘Lots of compounds are known to inhibit things like efflux transporters in the membrane of gram-positive and gram-negative [pathogens],’ explains Brendan Gilmore, professor of pharmaceutical microbiology at Queen’s University Belfast, UK. ‘What’s interesting here is this is a specific LPS-transporter system with seven proteins and the target is what they describe as a composite binding site. That means that the binding site is only present when the LPS is in the transporter itself and that’s when the compounds acts. It’s an elegant and neat approach.’
Why is this an important discovery?
Crab is a major global pathogen and is rated a priority 1 pathogen by the World Health Organization, alongside multidrug-resistant bacteria, and poses a particular threat in hospitals, nursing homes and among vulnerable patients. These pathogens can cause severe and even deadly infections.
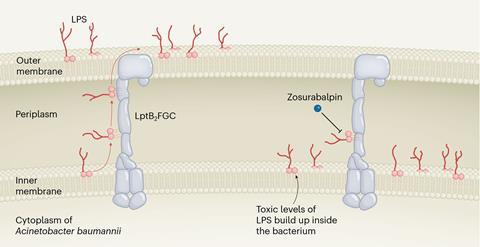
‘They are typically pathogens that are causing an increase in infections that are antibiotic resistant and have few or indeed no compounds in the pipeline to effectively tackle them, so that bumps them up the priority list,’ explains Gilmore. ‘[Crab] also has a very high fatality rate.’
Treatment options for Crab are declining, with mortality estimates ranging from 40 to 60%, in part due to the lack of effective treatment options. It is hoped that this new class of antibiotic could help. The discovery may also open the door to targeting the LPS-transport system of other gram-negative bacteria, such as Escherichia coli.3
How long has it been since we had a new class of antibiotics?
New classes of antibiotics do not come around very often. It has been over 50 years since a new antibiotic chemical class with activity against A. baumannii was approved.
‘There was a period of intense activity from the 1940s, through to the mid-1960s – the golden era of antibiotic discovery – and from that time we seem to have significantly reduced our ability to find new compounds … and we’ve kind of relied on those compounds ever since,’ says Gilmore.
Why is it so difficult to develop new antibiotics?
Gram-negative bacteria are particularly difficult to kill because their cytoplasmic membrane is surrounded by an outer membrane that blocks the entry of most antibiotics.
‘Gram-negative organisms decorate their outer membrane with LPS and that acts as a line of defence and gives inherent resistance against antibiotics and biocides,’ says Gilmore. ‘That’s why A. baumannii is a persistent hospital-associated pathogen, because it is resistant to many biocides that are used to sanitise high-contact surfaces.’
However, a major issue hindering the development of new antibiotics is the lack of incentives available to encourage large pharmaceutical companies to invest in antibiotics, rather than focusing on blockbuster drugs.
‘The business case for new antibiotics has essentially evaporated and although clinically the value placed is high, financially the value placed is reasonably low … and the major pharma companies have steadily exited the field,’ explains Gilmore. ‘Antibiotic discovery is difficult,’ he continues. ‘It’s all or nothing. You can invest a lot of time in it and discover nothing at all. The attrition rate for those compounds is high and, if they do get approval, companies are faced with that reality that [their drug] is likely to be subject to fairly strict antimicrobial stewardship.’
How close are we to having a new drug?
We now have one clinical candidate derived from this macrocyclic peptide class called zosurabalpin.2 The researchers found that zosurabalpin could treat highly drug-resistant Crab both in vitro and in multiple mouse infection models, including sepsis.
The researchers said the findings demonstrate the potential of zosurabalpin as an antibiotic and it has now been evaluated in two phase I clinical trials that showed single intravenous doses of 10mg to 2000mg zosurabalpin were safe and, overall, well tolerated.4 Additional clinical trials will be required to further assess the safety and efficacy of the drug before it can be considered for approval by drug regulators.
This new discovery has made headlines – but is it still too soon to get excited?
Even if zosurabalpin performs well in clinicals trials it will still need to go through the usual approval processes before it can be offered routinely to patients. Funding will also be needed to ensure this process can go ahead – and bringing a new drug to market is not cheap.
However, Gilmore highlights that this is a new compound for a ‘really problematic superbug’ with the weight of a major pharmaceutical company, Roche, behind it. He says the discovery of this new class of antibiotics should provide ‘hope’ that there are new targets out there and this may reinvigorate the antibiotic drug pipeline.
When might it be available for patients?
‘Five to 10 years would not be an unreasonable time frame to get from molecule to bedside,’ says Gilmore. ‘But this is when the big money comes in. There’s clearly a lot of resources that have been expended to date but this is when it gets real, and the big expense for Roche will come into play.’
References
1 C Zampaloni et al, Nature, 2024, DOI: 10.1038/s41586-023-06873-0
2 KS Pahil et al, Nature, 2024, DOI: 10.1038/s41586-023-06799-7
3 KG Morgan and PJ Hergenrother, Nature, 2024, DOI: 10.1038/d41586-023-03988-2
4 A Guenther et al, Open Forum Infect. Dis., 2024, DOI: 10.1093/ofid/ofad500.1749












No comments yet