The 2025 Nobel prize in physiology or medicine has been awarded jointly to three scientists – two based in the US and one based in Japan – for their discoveries regarding the role of regulatory T cells in keeping the immune system in check. Faulty regulatory T cells can result in autoimmune disease and as a result there has been considerable interest in this system from drugmakers.
The Nobel committee awarded the prize to Mary Brunkow, at the Institute for Systems Biology, in the US, Fred Ramsdell, at Sonoma Biotherapeutics in the US, and Shimon Sakaguchi at Osaka University in Japan ‘for their discoveries concerning peripheral immune tolerance’. Their discoveries have laid the foundation for a new field of research and led to the development of new treatments for autoimmune diseases and cancers.
Sakaguchi’s work began in the early 1980s, investigating the process that prevents the immune system from harming the body, a question that many researchers had tried and failed to answer. In his research, he removed the thymus from three-day-old mice, who then developed autoimmune disease, injected them with mature T cells developed in genetically identical mice and found that the T cells protected the mice from autoimmune disease.
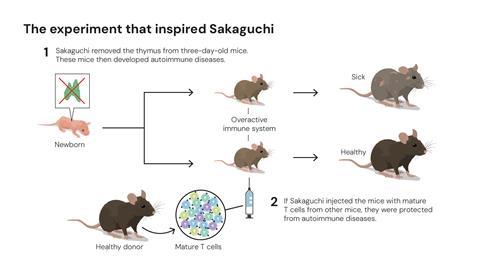
This convinced Sakaguchi that the immune system must have a security guard, of sorts, which helped to calm down other T cells in the body, preventing them from attacking the body. At the time, scientists already knew about helper T cells and killer T cells so he concluded that there must be another kind of T cell at work.
The work to find this other T cell took him over a decade, but in 1995 he presented regulatory T cells for the first time, showing that they were characterised by carrying CD4 and CD25 proteins on their surface.
Brunkow and Ramsdell were working at a biotech company, Celltech Chiroscience, in Washington, US , in the 1990s and had a particular interest in a mouse strain called ‘scurfy’. In these mice the males were born with scaly, flaky skin, enlarged spleen and lymph glands, and a lifespan of just a few weeks.
It was known that scurfy was linked to the X chromosome and was caused by T cells destroying the mice’s tissues, but Brunkow and Ramsdell wanted to understand the molecular mechanism underlying the disease. This required mapping the entire X chromosome in detail, narrowing it down to 20 potential genes. Eventually they discovered the faulty gene responsible for scurfy and, due to its similarities with FOX genes, called it Foxp3.
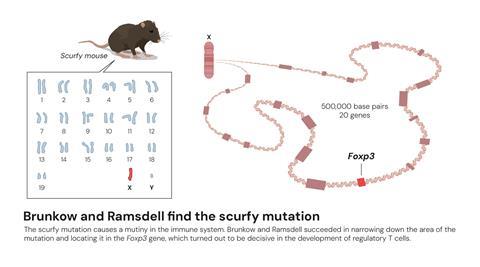
They went on to discover a human version of the Foxp3 gene, which was mutated in individuals with the X chromosome-linked autoimmune condition IPEX and, in 2001, they published a paper in Nature Genetics revealing that mutations in the Foxp3 gene caused both IPEX in humans and scurfy mice.
Researchers went on to make the link between this discovery and Sakaguchi’s work on regulatory T cells, and two years later Sakaguchi, followed by others, proved that the Foxp3 gene controls the development of regulatory T cells.
The work of Brunkow, Ramsdell and Sakaguchi has led to the development of many new treatments and there are now over 200 clinical trials involving regulatory T cells.
















No comments yet