
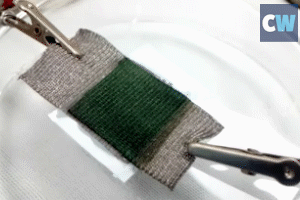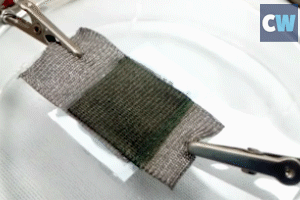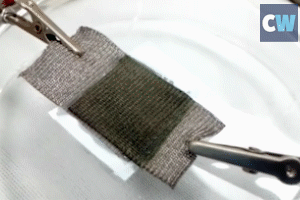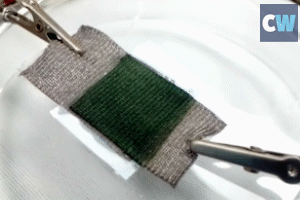
Bacteria can be genetically engineered to make the pigment that gives octopuses their camouflage powers, with yields up to a thousand times higher than traditional methods.
It is hoped that the plug-and-play approach could also be used to accelerate production of many other useful chemicals and materials, enabling the move away from fossil-fuel based materials.
The extraordinary ability of octopus, squid, cuttlefish and other cephalopods to change the colour of their skin to blend in with their environment has long fascinated scientists and non-scientists alike. The pigment responsible is xanthommatin – a structurally complex, colour-changing ommochrome with potential material and cosmetic applications. However, producing this pigment on a large enough scale to study it has proved challenging and expensive.
Aiming to come up with a more efficient and sustainable alternative, a research team, led by the Scripps Institution of Oceanography in the US, turned to bacteria. ‘No one wants to go up and extract a bunch of octopus for this pigment … it’s not a practical way to go about it,’ explains Bradley Moore, a marine chemist at Scripps. ‘Since it’s not a microbial compound, we felt that it would be a really cool opportunity to be able to turn a microbe to be able to make this material.’
The researchers developed a ‘growth-coupled biosynthetic strategy’ involving a feedback loop, whereby the survival of a bacterium – Pseudomonas putida – was engineered to be dependant (auxotrophic) on a byproduct of the pathway – formic acid.
P. putida, a soil bacterium, was chosen for its tolerance to xanthommatin, which is toxic to many microbial species, as well as its ability to encode the tryptophan-to-kynurenine pathway, which is required for xanthommatin production.
They designed and constructed a 5,10-methylenetetrahydrofolate auxotroph in P. putida and further modified the bacterium to have a critical deficiency that could only be relieved by formate released during xanthommatin production. They did this by carrying out five genomic modifications to the parental strain, including the deletion of four genes and the insertion of a formate assimilation module.

‘We’re asking the microbe to make a material for us and at the same time we’re going to make sure that we give something to the microbe [so it will] want to make this material for us,’ Moore says. ‘At the end of the day, it doesn’t care about xanthommatin, it cares about formic acid; the formic acid becomes life for this organism .’
The strategy meant that for every molecule of pigment generated, the cell also produced one molecule of formic acid, which then provided fuel for the cell’s growth. ‘The more formic acid it makes, the better it grows, because we made it an auxotroph for that material. Most organisms like to have ATP. And the more ATP you make, the more xanthommatin you make – it became beautifully coupled.’
Bacterium is a master of cephalopod pigment synthesis
While traditional approaches to synthesising xanthommatin yield around 5mg per litre, the new method was found to have a yield over a thousand times higher, reaching between 1–3g per litre. The approach can also be applied to the production of other useful materials, although the group are yet to publish these findings. ‘We’ve trained the organism primarily to work on formic acid right now, but as you can imagine, there are many other forms of C1 metabolism as byproducts, and now we’re expanding out to all those as well … We see thousands of pathways being opportunistic in this way.’
Xanthommatin’s optoelectronic properties mean it has potential in applications ranging from photoelectronic devices, thermal management coatings, dyes and ultraviolet protectants. Moore says that the team’s material scientists are keen to take xanthommatin and make devices that can change colour in response to different stimuli.
Florent Figon, an expert in pigment biochemistry and ecology at the Grenoble Alpes University in France, describes the study as ‘very clever’. ‘There are indeed lots of difficulties to synthesise xanthommatin in a fair amount, even more with a process close to in vivo conditions,’ he notes. ‘Here the authors show a robust way to perform it in a scalable way. Although I am not sure how it could be used by other labs, since it seems to require specific tools and skills.’
Figon says that the xanthommatin biosynthesis pathway was first unravelled around the middle of the 20th century but the compound has only been synthesised in recent years. ‘However, the process is quite tedious, expensive and produces a few milligrams to grams of not-so-pure product,’ Figon says. ‘Furthermore, ommochromes are well-known to be hard to analyse by NMR so we need it in greater amount than other compounds.’
References
L B Bushin et al, Nat. Biotechnol., 2025, DOI:10.1038/s41587-025-02867-7








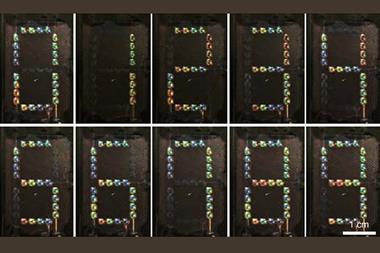






No comments yet