Scientists in France and Switzerland have performed molecular dynamics simulations to understand why some natural materials are able to remove heavy metal ions from contaminated water. By unravelling these microscopic mechanisms, the team hope to yield insights that others can use when designing water purification systems.
Complex, but often expensive, technologies for treating water exist, such as membrane filtration, activated carbon adsorption and electrocoagulation. Bio-based materials are also showing great promise as low cost adsorbents. Many of these are materials that would otherwise be thrown away and include nuts, fruit and vegetables peelings, seaweed, spent coffee grounds and tea leaves. A 2018 study from researchers in India used spent coffee grounds to remove lead and fluoride from contaminated water with an efficiency of around 90%.2 And in 2020, a team in Turkey showed that unmodified tea waste could remove four different heavy metals from water.3 Despite overwhelming evidence of their performance, natural materials have yet to be used on a large scale.
‘[The materials] are cheap, abundant and environmentally friendly. However, there has, hitherto, been little work describing attempts to experimentally characterise the microscopic nature of these natural trapping agents and related microscopic mechanisms. Why this contrast? Certainly, the complexity of these natural materials is one reason. For example, they each consist of thousands of different chemical components,’ comments Wanda Andreoni, from the Swiss Federal Institute of Technology in Lausanne (EPFL).
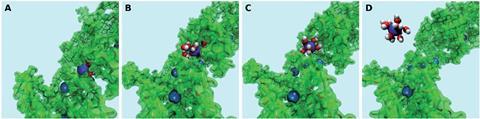
Now, Andreoni and her colleagues have taken the first step towards understanding the capturing action of such materials by using simulations at the molecular level for the first time in this field. ‘We used state-of-the-art simulations that we have previously applied to a variety of systems and physicochemical processes,’ explains Andreoni. They decided to simulate how hemicellulose captures lead ions from water, as the polysaccharide occurs in many plant-based materials. Their results established that hemicellulose could easily take up lead ions from water via adsorption, whereas it was energetically expensive to release the ions back into water. They also reported a dual role for water, as a competitor of the polymer for the uptake of the metal ions, as well as a participant in the bound metal–polymer states.
Annabella Selloni of Princeton University in the US, who develops computational tools to understand surfaces and interfaces for applications that include environmental remediation, says the research ‘provides a clear, detailed and convincing picture of a complex physical process of great relevance to the problem of water purification from toxic metals.’ She comments that the researchers combined clever modelling with several advanced computational methodologies, from first principles calculations and classical molecular dynamics to enhanced statistical sampling approaches. ‘In particular, key ingredients of the study are the collective variables used for the metadynamics simulations, which are effective descriptors essential for a correct analysis of the process. Using only two uniquely suited collective variables, the researchers were able to classify the different configurations of their system of several thousand atoms, distinguish the configurations where the metal ions act as contaminants in water from those where they have been captured and trapped by the organic material, and even provide quantitative estimates for adsorption/desorption barriers. All this information can be most useful for the design of water purification systems based on natural materials.’
The team is keen to draw scientists from different fields towards environmental remediation. ‘We believe that fundamental research should focus on crucial problems for society and it is high time to start and make use of state-of-the-art methods, both experimental and theoretical,’ comments Andreoni. ‘Support to our conviction comes from a similarly challenging field of research: medicinal chemistry and drug design. Biological systems on which drug design focuses are certainly not less complex than say spent coffee or banana peels. Still simplified model systems combined with computational methods – especially molecular dynamics – have played a very important role for progress in drug discovery. These same approaches should be used to tackle environmental problems.’
‘Water purification is one of the most important challenges of the 21st century, as a large portion of our global population has no access to clean water,’ comments Wendy Lee Queen from EPFL, who has previously developed MOFs for water treatment, but wasn’t involved in this study. ‘Unfortunately, at the moment, there is little understanding of how adsorbents function in the extraction of a given contaminant from water. This work could help accelerate the design and discovery of optimal adsorbents for the extraction of highly toxic contaminants from water and make the water purification process more sustainable and accessible to all.’
References
1 F Pietrucci, M Boero and W Andreoni, Chem. Sci., 2021, DOI: 10.1039/d0sc06204a (This article is open access.)
2 A N Babu et al, J. Environ. Manage., 2018, 218, 602 (DOI: 10.1016/j.jenvman.2018.04.091)
3 H Çelebi, G Gök, O Gök, Sci Rep., 2020, 10, 17570 (DOI: 10.1038/s41598-020-74553-4)










1 Reader's comment