Scientists have developed a class of very bulky ligands that can be used instead of traditional N-heterocyclic carbene ligands in various transition metal-catalysed reactions.
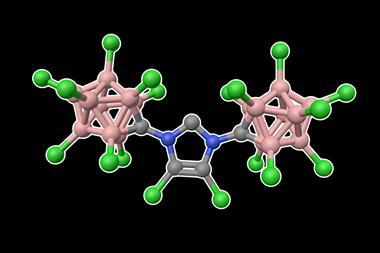
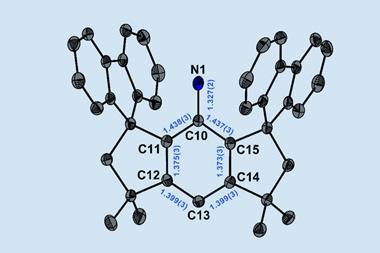
N-alkyl N-heterocyclic carbenes (NHCs) are cyclic carbene compounds typically containing two nitrogen atoms. Compared to other carbenes, NHCs are stabilised both electronically through orbital overlap and sterically through the presence of bulky groups on the nitrogen atoms. They can form strong bonds with metals, leading to stable complexes that catalyse a wide range of reactions. One of the most useful NHC ligands is 1,3-di-tert-butylimidazol-2-ylidene (ItBu), which has tert-butyl groups on each nitrogen atom. Changing the tert-butyl groups to tert-octyl groups would significantly increase the steric volume, and therefore the stability, provided by these side groups.
That’s exactly what a team, led by Michal Szostak of Rutgers University in the US, has done. The tert-octyl ligands (ItOct) they have synthesised have the same strong σ-donating and π-accepting abilities as ItBu but much bulkier tert-octyl side chains. ItOct has the highest steric volume of N-alkyl N-heterocyclic carbenes reported to date. They are also relatively straightforward to make, using readily-available tert-octylamine as the starting material. Condensing tert-octylamine with glyoxal then cyclising the diimine gave the desired ItOct as the hydrochloride salt.
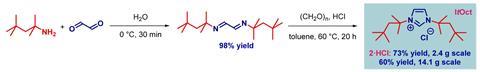
Szostak’s team formed catalytic complexes by combining the new ligands with transitional metal ions, which they tested in a range of reactions to demonstrate the benefits of these bulky side chains. The [ItOct–M] complexes out-performed the equivalent [ItBu–M] complexes in reactions including Au(I)-catalysed hydration and Pd(0)-catalysed C–C coupling. The ligands are now commercially available and the research team is continuing to investigate their ability to stabilise reactive metal centres.









No comments yet