An unprecedented four-electron carbon intermediate has been isolated and characterised by researchers in the US. The team used a two-step approach to remove the non-bonding electrons from a stable carbene intermediate to create a crystalline doubly oxidised carbene.
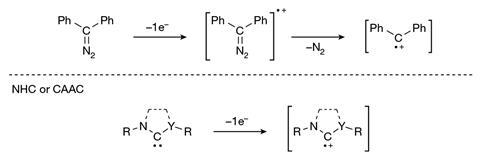
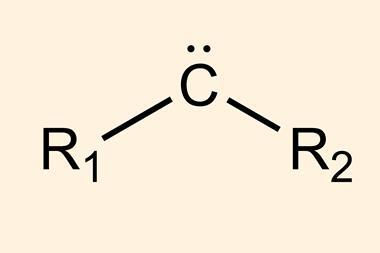
Carbenes were first discovered at the beginning of the 20th century but their extreme reactivity made them difficult to handle and they were widely dismissed as a laboratory curiosity. These reactive species contain an unusual six-electron carbon atom, bonded to two substituents and with two non-bonding electrons on the carbon centre. Careful choice of the bonded groups can stabilise this electron-deficient carbon and over the last 30 years carbenes have become a powerful tool in synthesis.
Now, Guy Bertrand and Ying Kai Loh at the University of California San Diego, US have pushed the octet rule of main group elements – that atoms bonded to one another tend to have eight electrons in their valence shell – even further, removing the two non-bonding electrons to prepare a highly-reactive four-electron carbon species. The team began with a bis(imino)carbene whose two electron-donating substituents stabilise the vacant orbital on the central six-valent carbon. However, they quickly encountered problems using single-electron oxidation conditions.
The initial oxidation produces an unstable five-electron carbene radical cation which immediately abstracts a hydrogen radical from the reaction solvent to form an unwanted side product. ‘We therefore employed a two-step approach,’ explains Loh. ‘First, oxidation of the carbene to a carbonyl compound, then, oxide-ion abstraction which removes the oxygen atom along with two electrons to afford the doubly oxidised carbene. This approach bypasses the generation of that highly reactive carbene radical cation.’
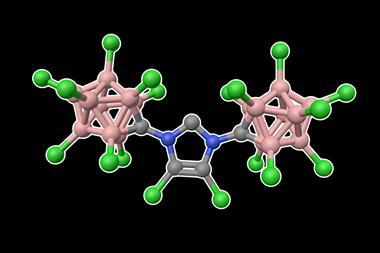
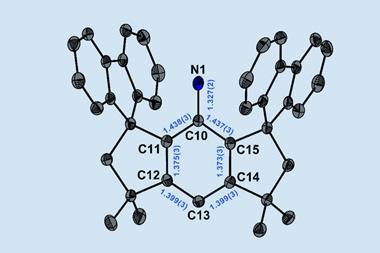
The resulting crystalline dication contains just four valence electrons, but the team’s careful choice of bulky electron-rich substituents protects the electron-deficient carbon centre from quenching by the crystal counterions while simultaneously stabilising the double positive charge. ‘The authors cleverly used imine functional groups to assist in this stabilisation,’ explains Todd Hudnall, a main group chemist at Texas State University in the US. ‘The two flanking nitrogen atoms are electron rich and feature lone pairs of electrons that are capable of stabilising the formal C2+ centre through resonance effects. In such a way, the 2+ charge can be distributed throughout the N–C=N–C–N=C–N linkage.’
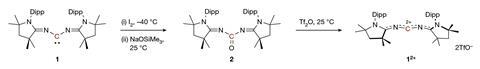
Even with this stabilisation, the doubly oxidised carbene is potently electrophilic, reacting via the central carbon as both a Lewis acid and an anion abstractor. Intriguingly, the team also identified reduction conditions to convert the dication back to the parent carbene, making the interconversion of these species a unique, reversible two-electron redox system.
Bertrand is confident that this new reactive species will ultimately find many uses throughout the chemical and material sciences and hopes that others will begin to explore the potential of these carbenes. ‘This work is fundamental in nature but it paves the way for the isolation of a variety of doubly oxidised carbenes,’ he says. ‘In the short term, we need to demonstrate that this compound is not unique and many dications could be stable with simpler substituents. In the future other applications may arise and I have no doubt that we or others will find them.’
This discovery has already met with excitement and Hudnall is particularly eager to see how this work will influence future catalytic strategies. ‘Given the highly delocalised nature of the 2+ charge in the molecule, it would be interesting to see if this dication can be used to activate small molecule substrates relevant to the renewable energy arena such as carbon monoxide, carbon dioxide or hydrogen,’ he says.
References
YK Loh et al, Nature, 2023, DOI: 10.1038/s41586-023-06539-x












No comments yet