Surprising ‘aerogen bonding’ completes set of p-block groups exhibiting the phenomenon
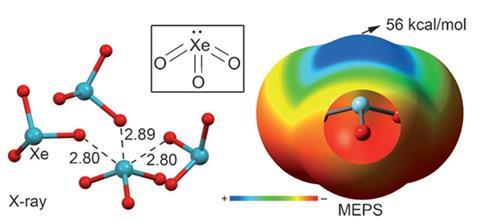
Despite noble gases’ characteristic unreactivity, Spanish chemists have calculated that molecules containing xenon can interact non-covalently through what they’ve called ‘aerogen bonding’. The electrostatic potentials that Antonio Bauzá and Antonio Frontera from the University of the Balearic Islands in Palma computed can help explain such molecules’ crystal structures.
Aerogen bonds involve low electron density areas around xenon atoms, known as s-holes, which have already been found to mediate non-covalent bonds for every other p-block group. Frontera and Bauzá previously identified similar interactions occurring with group 14 elements like silicon, dubbing it ‘tetrel bonding’, continuing the tradition of incorporating the group’s trivial name. Likewise, s-hole interactions involving group 15, 16 and 17 elements are known as pnicogen bonding, chalcogen bonding and halogen bonding respectively. According to Frontera, discovering that such phenomena occur in group 18 noble gases gives ‘a universal sense’ to the s-hole concept, which is becoming important in supramolecular chemistry and biochemistry.
Xenon trioxide was one of the molecules the chemists studied. ‘We performed several computational analyses that showed a region of positive potential over the xenon atom, located on the extension of the xenon–oxygen bond,’ Frontera explains. Consequently positioned on the opposite side of the xenon atoms to the bond, along the same axis, the positive charges let the atoms act as Lewis acids. These s-holes are therefore attracted to Lewis base electron pairs on oxygen atoms in other xenon trioxide molecules. Bauzá and Frontera found aerogen bond energies to be similar to hydrogen bonds and other s-hole interactions but less directional. The resulting attraction is likely the reason why xenon and oxygen atoms appear closer than otherwise expected in x-ray crystallography studies of xenon trioxide.
‘I really like this work because it shows that chemistry is full of surprises,’ says Stefan Zahn from Justus-Liebig-University Gießen, Germany, who was part of the team that identified pnicogen bonding. ‘It is quite unexpected that noble gas atoms can form non-covalent interactions of comparable strength to hydrogen bonds.’
Frontera concedes that, due to the rarity of molecules containing noble gas elements, aerogen bonding ‘is not expected to play a significant role in supramolecular chemistry’. However, he hopes it could be important in xenon chemistry, and also underlines the theoretical importance of showing the generality of s-hole interactions. His team is now exploring what other types of unusual bonding interactions xenon might form, and also studying aerogen bonding in argon and krypton.











No comments yet