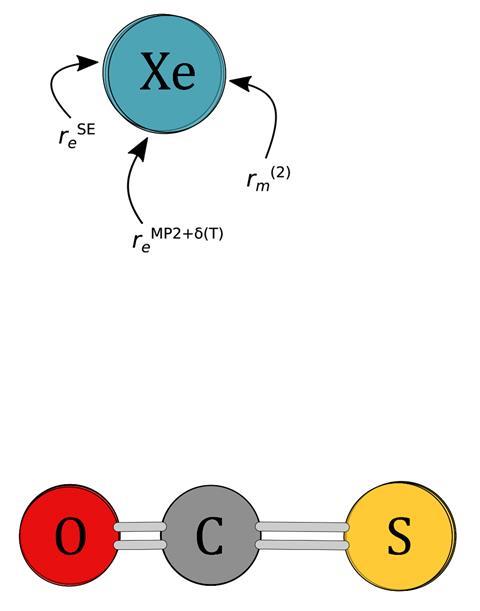
Scientists calibrating their instruments have managed to characterise an unusual noble gas complex. Using quantum chemical calculations and rotation spectroscopy, they have unpicked the structure of the non-covalent xenon⋯carbonyl sulfide complex (Xe⋯OCS) and established a benchmark-quality reference for this rare gas interaction.
The researchers from the Leibniz University Hannover in Germany first noticed the Xe⋯OCS complex in their spectra when calibrating their microwave spectrometer. They thought they were measuring the spectrum of OCS; it soon transpired that the bottle of OCS had been mixed with xenon. The complex isn’t chemically bonded but is held together through weak non-covalent interactions, which had made predicting its structure difficult.
‘Computational chemistry usually gives you the equilibrium position, without any vibrational effects, while experiments almost always give you structures that are vibrationally averaged,’ explains Peter Kraus who worked on the computational elements of the research. ‘We were lucky with xenon because it has so many isotopes. You can use experimental data to back-correct for this energy difference and compare structures directly.’

‘Once you have the structure, you can predict the spectrum, then the more accurate your structure the closer your predictions are to the actual spectra and this saves a lot of instrument time,’ says Kraus. To ensure that they obtained the most accurate structure possible, the scientists included relativistic effects in their calculations.
‘This work highlights the necessity of reliable experimental data for the correct benchmarking of theoretical quantum chemical calculations,’ comments Sonia Melandri, who studies the structure of molecules and weakly bound complexes using spectroscopy and theoretical methods at the University of Bologna in Italy. ‘By bringing together accurate structure determination obtained from microwave spectroscopy experiments and extensive quantum chemical calculations, the driving forces governing the formation of the Xe⋯OCS complex can be critically evaluated.’
‘Clearly, intermolecular interactions are of great importance, yet there is still much to learn from simple systems,’ adds Helen Leung, who researches interactions between non-chemically bonded molecules via rotational spectroscopy at Amherst College in Massachusetts, US. ‘Although there is no substitute for a complete experimental characterisation of a system, sometimes this is not available. This study beautifully illustrates how experiment and theory can complement each other and work together in advancing our understanding of chemical systems.’
By combining this latest finding with existing structure predictions for non-covalent complexes, Kraus hopes to create a database of valuable reference data that can train new functionals (mathematical expressions to describe the electron density of a system) and help scientists understand systems with similar interactions.
References
This article is open access
P Kraus et al, Phys. Chem. Chem. Phys., 2020, 22, 5615 (DOI: 10.1039/d0cp00334d)









No comments yet