
Molecular electronics can help to extend Moore’s Law – the prediction that the number of transistors on integrated circuits will double approximately every two years – beyond the physical limits of silicon devices. It involves incorporating functions and properties of traditional semiconductors (such as transistors and diodes) into single molecules, providing advantages such as small size, low cost and being able to tailor device properties.
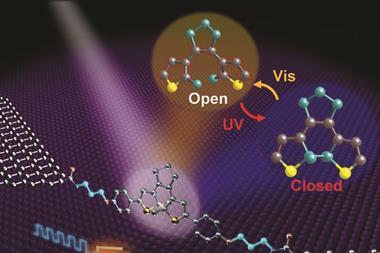
Stéphane Rigaut and Xiaodong Chen and their colleagues from University of Rennes 1, France, Nanyang Technological University, Singapore, and University of Mons, Belgium, have demonstrated and rationalised reversible switching in a molecular transport junction (structures in which molecules conduct electrical current between two electrodes) based on organometallic wires and nanogaps, an important step towards realising molecular-scale devices.
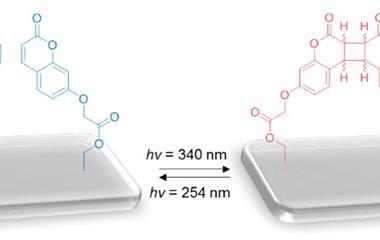
Their photoswitching device involves dithienylethylene molecules linked to two gold electrodes with two [HS–C6H4–CC(dppe)2Ru]+ (dppe being 1,2-bis(diphenylphosphino)ethane) side groups. Upon irradiation with UV light, the dithienylethylene cyclises into a ‘closed’ state that conducts electricity and returns to its ‘open’ non-conducting state with visible light. Although this property is well documented in solution, Rigaut’s work is a rare example of reversible switching in the solid state.
‘[Target properties of molecules] are first evaluated in solution for convenience,’ explains Rigaut. ‘For potential applications, a major aspect deals with the problem of integration of molecules in solid state nanoscale devices, in which properties observed in solution might be altered.’
The researchers’ device was able to achieve reversible switching because the ruthenium fragments decrease the coupling of dithienylethylene with the metal electrodes, allowing the molecule to close upon UV irradiation.
Paul Low, an expert in molecular electronics at the University of Durham, UK, praises the progress made in this work. ‘The thing that really makes this interesting is that it’s taking the organometallic systems into the device platforms,’ he says. He adds that ‘it provides the evidence that transition metal containing molecules of significant complexity can be assembled within junctions’.
The researchers are now looking to make a device using molecules that can show more than two different states, allowing more complex and functional devices.
References
- F Meng et al, Chem. Sci., 2012, DOI: 10.1039/c2sc20323e







No comments yet