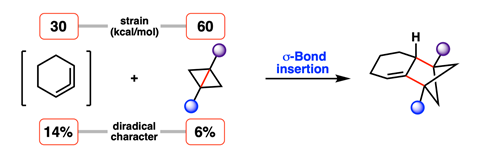
A mild and simple method to access an important pharmaceutical scaffold, the bicyclohexane motif, has been developed using only the innate reactivity of two strained substrates. The new reaction proceeds via an unusual σ bond insertion, exploiting the diradical-like character of the starting materials to drive the reaction without activation.
Aromatic rings are common in drug compounds, largely owing to their ease of synthesis and manipulation. However, undesirable properties arising from these flat, unsaturated structures mean that increasingly the pharmaceutical industry is seeking to replace these motifs with rigid 3D frameworks. Bicyclo[2.1.1]hexanes (BCHs) are an important structural mimic of di-substituted aromatics and this scaffold has already been incorporated into several promising drug candidates.
‘BCHs are most commonly made by reactions of unstrained alkenes with bicyclobutanes (BCBs),’ explains Neil Garg, an organic chemist at the University of California, Los Angeles. ‘Known reactions typically require an external stimulus, such as light or a radical initiator, to trigger a single-electron reaction mechanism between the alkene and BCB.’ However, Garg and long-term collaborator Kendall Houk hypothesised that by harnessing the masked reactivity of two strained substrates, bicyclobutanes and cyclic allenes, they could access this complex motif activator-free.
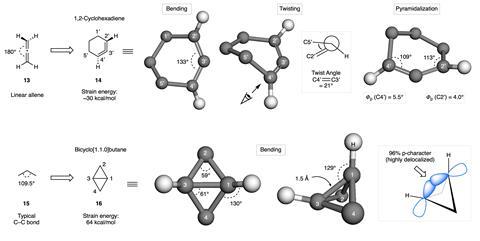
BCBs have an impressive strain energy of around 64kcal/mol, more than twice that of cyclopropane, as the fused ring system compresses the bridgehead carbon–carbon bond to just 61°, compared with 109.5° for an unstrained bond. ‘There’s a geometric distortion there which means the orbital overlap is weaker, so the bonds are weaker,’ explains Johannes Walker, a synthetic chemist at the University of Göttingen in Germany. ‘You end up with more diradical character so that the electrons aren’t quite so close to each other.’ Likewise, the expected linear geometry of the cyclic allene is restricted to just 133°, simultaneously twisting the structure to induce radical-like behaviour.
Garg’s team used a silyl triflate precursor to generate the unstable allene in situ, this transient reagent immediately reacted with the BCB substrate at room temperature to form the expected bicyclohexane product. They then investigated the activation-less mechanism behind this sigma bond insertion using computational and modelling studies.
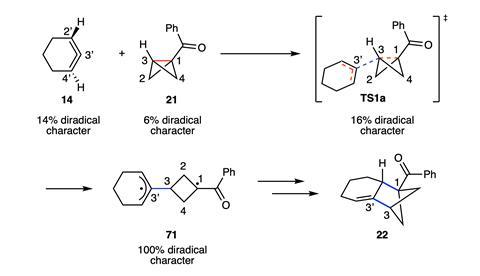
According to their calculations, the cyclic allene possesses 14% diradical character and attacks the BCB substrate at the bridgehead, breaking this bond to form a 100% diradical intermediate. The two resulting radical centres, one on the allene unit and one on the cyclobutane, then recombine to create a second C–C bond, formally inserting two σ bonds to produce the BCH product. The inherent diradical character in both substrates, coupled with their geometric distortion towards the transition state, accounts for the barrierless reaction, the team explains.
‘The use of twofold strain release driven reactions is a very elegant way to achieve a two carbon insertion into BCBs and totally different from other work in this field where activators are often used,’ says Ed Anderson, an organic chemist at the University of Oxford. ‘I could see this work therefore being of high appeal to the pharmaceutical industry, as well as being of intrinsic interest from a reactivity perspective.’
The team hope that medicinal chemists will leverage this reaction to access more complex drug scaffolds. They also believe that this unusual reactivity could be harnessed by organic chemists in the development of new methodologies and Garg’s group are currently working to expand this diradicaloid chemistry to other substrates.
For Walker, it’s this alternative perspective on radical chemistry that is the most interesting and novel aspect of the work. ‘The concept of taking two fragments that have this diradical character and putting them together really could be quite exciting. The challenge going forward is finding more fragments that have this behaviour,’ he says.
References
AT Meza et al, Nature, 2025, DOI: 10.1038/s41586-025-08745-1














No comments yet