The formation and structural dynamics of molecular ions in the gas phase have been monitored for the first time using high-speed electron diffraction combined with resonance-enhanced multiphoton ionisation. The new method overcomes some of the difficulties associated with exploring these chemical species, unlocking exciting opportunities in ion chemistry.
‘This remarkable achievement opens up a new frontier in imaging, offering a paramount contribution to our understanding of the fundamental mechanisms governing chemistry,’ says Andrea Trabattoni at DESY Hamburg and Leibniz University Hannover in Germany, who wasn’t involved in the study. He calls the approach groundbreaking and says that it holds promise for extension to other systems.
Molecular ions in the gas phase play important roles in many reactions, especially in atmospheric and interstellar chemistry, but studying these species and watching how they form and change in real time has been tricky. ‘Ions are highly reactive in their natural state, seeking stabilisation through interactions with other species. This inherent property makes it challenging to observe them,’ explains Jun Heo from the Institute for Basic Science (IBS) and the Korea Advanced Institute of Science and Technology (KAIST) in Daejeon. ‘Our approach allows us to capture the moment when ions undergo changes in a video format.’
‘Our study is the first to observe the dynamics of ions in the gas phase,’ says Hyotcherl Ihee, who also works at IBS and KAIST. ‘In our experiments, we used SLAC’s mega-electronvolt ultrafast electron diffraction (MeV-UED) instrument, one of the devices capable of generating the fastest electron pulses in the world.’ Ihee’s colleague, Doyeong Kim, adds that the facility can accelerate electrons to 0.99 times the speed of light.
The researchers studied the fate of 1,3-dibromopropane cations (DBP+), which they generated using resonance-enhanced multiphoton ionisation (REMPI). ‘Conventional ionisation methods are often radical, leading to the fragmentation of the target system or the generation of unwanted byproducts,’ says Kim. ‘REMPI, on the other hand, is a very gentle method. It selectively creates the specific ions we aim to observe.’
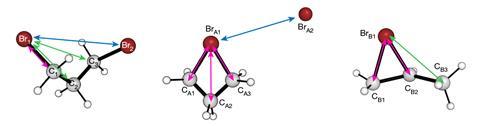
‘The ions are prepared on the femtosecond timescale by photoionisation of neutral molecules,’ explains Thomas Wolf, a scientist at SLAC who didn’t take part in the research. ‘Using ultrashort electron pulses at picosecond delays with respect to the photoionisation, structural snapshots can be measured while the ions are still highly concentrated and before they had enough time to fly apart.’
‘Typically, when ions are forcibly created, it is anticipated that they will rapidly undergo structural changes or break into different forms, but our results revealed a phenomenon contrary to these expectations,’ points out Heo. He explains that shortly after generation of the DBP+ ions, there is a pause so that the structural changes are not immediately apparent. The ions remained in this ‘dark state’ for 3.6 picoseconds and then gradually decayed to the most stable configuration, which was a bromonium ion with a three-membered ring structure.
Wolf says that although the DBP+ system is comparably simple, due to the strongly scattering bromine atoms in the ion and the REMPI wavelength being readily available from standard laser systems, the facility at SLAC now has advanced wavelength tuning capabilities, so he believes that experiments on a larger class of molecular ions could become feasible.
‘To apply this method to a more universal ion system, it is necessary to devise various techniques, such as effectively vaporising the sample and ionising it,’ adds Ihee. ‘Therefore, we believe that additional innovations are needed for these methods to be more widely adopted, but if this happens, we anticipate that our approach could be used to study a variety of known chemical substances.’
References
J Heo et al, Nature, 2024, DOI: 10.1038/s41586-023-06909-5










No comments yet