Chemical bonds are part of the way chemists rationalise the behaviour of atoms in the conditions of the world around them. Tim Wogan looks at how they are affected when those conditions change
‘The only criterion of a model is usefulness, not its “truth”,’ wrote the theoretical organic chemist Michael Dewar in 1984. At root, electrons obey the laws of quantum mechanics and atoms normally move into whatever geometrical configuration minimises the energy of their quantum wavefunction. However, the Schrödinger equation is analytically soluble only for the hydrogen atom, and its numerical solution is computationally unfeasible for large systems. For over a century, therefore, chemists have categorised certain types of atomic interactions as ‘bonds’ distinct from, say, van der Waals’ interactions. These distinctions are, however, heuristic: Iupac’s definition of a chemical bond states simply that ‘there is a chemical bond between two atoms or groups of atoms in the case that the forces acting between them are such as to lead to the formation of an aggregate with sufficient stability to make it convenient for the chemist to consider it as an independent “molecular species”.’
Following this principle, chemists have also found it convenient to sub-divide chemical bonds into types such as covalent bonds (in which the bonding electrons are localised near the atoms of the bond and the electronic charge is divided approximately equally between them), ionic bonds (in which the charge is also localised, but resides largely on one, more electronegative atom) and metallic bonds (in which the bonding electrons all enter delocalised conduction bands that permeate the material). Regardless of their mathematical inexactitude, such concepts generally work well under the conditions at which everyday chemistry is observed and conducted. They explain why ionic compounds usually dissolve in polar solvents like water to produce conductive liquids, whereas giant covalent structures are less soluble. They explain the ductility and electrical conductivity of metals.
But models that are ‘useful’ under one set of conditions are not necessarily so under others, and chemical bonding can look very different at unfamiliar temperatures and, especially, pressures.
Hot and cold
When physicists wish to understand chemistry at a fundamental level, they often dial down the thermostat. Near absolute zero, the thermal motion that blurs interactions at ambient temperatures is almost stilled and their quantum nature comes into focus. John Doyle, an ultracold atomic physicist at Harvard University in Massachusetts, US, works to cool large polyatomic molecules to low temperatures.
Once the atoms are very close, the chemistry that occurs is the same as at room temperature
Doyle’s group recently succeeded in cooling calcium monohydroxide to 110μK, and is now looking to cool larger molecules. The idea, says Doyle, is to achieve single quantum state control of all the degrees of freedom of these complex molecules. ‘You can have vibration, you can have rotation, you can have spin, and it turns out there are even more complicated states you can use in a molecule,’ Doyle explains. Possible uses of this individual state control include quantum computing and exploration of the origins of matter–antimatter asymmetry. Nevertheless, for modelling the bonding itself, the traditional picture works well at ultracold conditions. ‘Once the atoms are very close the chemistry that occurs is the same chemistry that occurs at room temperature,’ Doyle says.
Raising the temperature causes these quantum subtleties to become less apparent. Substances behave variably when heated – some melt and then boil, whereas some simply decompose on heating. Eventually, as the temperature keeps rising, electrons become unbound by atoms. At this point, chemistry becomes impossible and matter enters the domain of plasma physics.
When matter is confined inside an external potential or a container, a rise in temperature is generally accompanied by a rise in pressure (for an ideal gas temperature and pressure are proportional, but an ideal gas by definition comprises non-interacting particles that therefore undergo no chemical bonding). And at elevated pressures chemical bonding gets weird.
The principal reason is that, according to the laws of thermodynamics, a substance at constant pressure adopts the configuration that minimises its enthalpy. This is the sum of the internal energy of the particles and the work the substance as a whole has to do against the confining pressure to maintain its volume. At ambient pressure, this second term plays a much lesser role in dictating a material’s structure: ‘If it wants to be a large, spongy, open material with lots of volume in it, it’s perfectly fine,’ says electronic structure theorist Chris Pickard of the University of Cambridge, UK. ‘An example might be the zeolites.’ At high pressure, however, such structures are strongly disfavoured. ‘As you try to make a substance more compact, it might do things with the atoms and with the electrons in the atoms that raise the internal energy,’ says Pickard. ‘So a normal chemist would look at it and say “Oh, that’s a horrible way to put the bonds,” but by doing that it’s gained a bit by becoming a bit denser.’
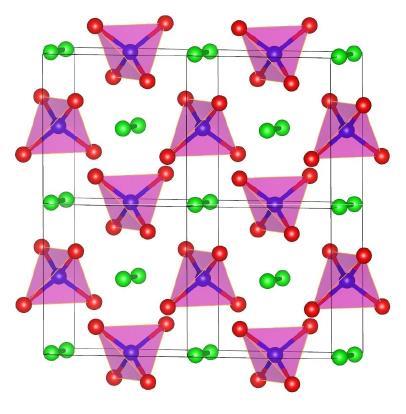
In 2011, for example, Pickard and colleagues calculated that, between 2.5TPa (about 25 million times atmospheric pressure) and 7.1Tpa, the most stable phase of nitrogen would comprise N2 dimers within a matrix of N5 tetrahedra. Two different models suggested that electrons would be biased strongly towards the tetrahedra, effectively forming an ionic salt in which the cation and the anion were the same element.
These pressures were above those experimentally accessible at the time, but Pickard – whose work, like that of other theoreticians, has modelled pressures orders of magnitude higher than this – remains confident of their reliability up until the point at which the nuclei are pressed so close together that the strong nuclear force comes into play. ‘The approximations behind density functional theory become more accurate at high pressures,’ explains Pickard. ‘Density functional theory works by saying that we don’t know the energy of an arbitrary arrangement of charge but we can do high-level quantum-mechanical calculations for uniform densities of charge at different densities… When you take a material and you squeeze the atoms closer and closer together, generally speaking you are making the charge density more uniform.’
Experimentalist Mikhail Eremets of the Max Planck Institute for Chemistry in Mainz, Germany, agrees: ‘We have a good experience with predictions,’ he says. Eremets and colleagues showed in 2004 that, at pressures above 110Gpa and temperatures above 2000K, diatomic nitrogen transforms to a single-bonded cubic form. If it could be quenched to ambient conditions, it would have much higher energy density than any other material and could find applications in rocket fuel, for example. Eremets intends to return to the topic, and others have subsequently shown that, once the triple bond is broken, nitrogen can display an extraordinary chemistry analogous to that of carbon.
Metallic mystery
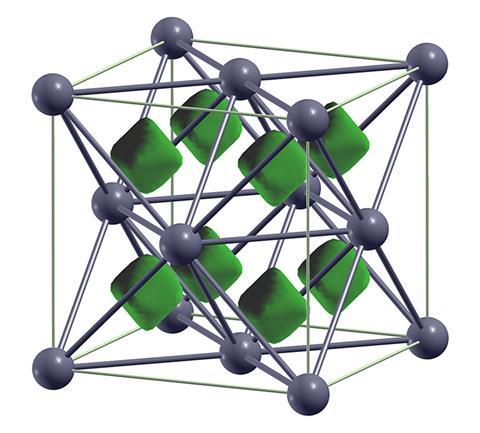
In 1926, John Bernal predicted that, at a sufficiently high pressure, all matter would become metallic as the internuclear distances would become smaller than the electron wavelengths, so all the electrons in the material would become free. This increasing electronic delocalisation is not straightforward, however. Notably, some metals actually become insulators at high pressure. In 2009, Eremets and leading high-pressure physicists from all over the world showed that, when placed in a diamond anvil cell, sodium became optically transparent at pressures of around 200GPa. This showed that a bandgap had opened, rendering the substance unable to absorb light below the gap. ‘This is an example of an electride, in which electrons are captured between two sodium ions,’ says Eremets.
Pickard says this kind of electride bonding is extremely rare under ambient conditions, but could be common at high pressure: ‘The electrons just gang together and sit in their own place in the crystal structure,’ says Pickard. ‘When you squeeze the atoms too much the electron is getting confined so much it’s costing too much kinetic energy, so it just gives up on that and goes and sits somewhere else.’
The maximum pressures attainable in diamond anvil cells are several hundred gigapascals, depending on the cell and the nature of the sample. To go higher than this – albeit momentarily – researchers sometimes use shock compression, in which a sample is rapidly compressed from all sides by simultaneous, powerful laser pulses. This does work on the material, causing the volume to decrease dramatically and the internal energy of the atoms or molecules to shoot up. Often too far up: chemists are usually most interested in the moderate temperature regime at which substances do not instantly melt or decompose. This is especially true when their main analytical tool is x-ray diffraction, as is the case for a sample that only exists for a small fraction of a second.
Researchers such as those using the world’s largest laser, the US National Ignition Facility (NIF) at Lawrence Livermore National Laboratory in California, have therefore pioneered an alternative technique called ramp compression. This involves a series of carefully-shaped shocks that compress the material in stages, allowing it to release energy from the previous compression before the next series of shocks hits. In 2014, researchers used NIF to ramp compress diamond to 5TPa without melting it.
Two years previously, Pickard and colleagues had published a paper predicting that carbon would undergo a series of phase transitions from diamond at high pressure starting with a transition to the BC8 structure at 1TPa and finishing in a body-centred cubic structure above 650TPa. ‘We had some problems getting that published because the referees were grumbling that it was completely irrelevant to life, the Universe and everything,’ says Pickard, ‘but it actually turned out to be incredibly relevant because the capsules they use at NIF are made mostly of carbon, so the people at Lawrence Livermore were incredibly interested. If you’re trying to ultimately compress carbon so you can compress hydrogen and do fusion, you kind of need to know what phase transitions might happen on the way so you can control the energy.’
In 2021, researchers at Lawrence Livermore successfully obtained x-ray diffraction data of a ramp-compressed diamond at 2TPa – the highest-pressure x-ray diffraction data ever obtained – and found that it had not undergone a phase transition. ‘The explanation we came up with is that the energetic barrier to transforming diamond to the BC8 structure is so high that, in a fast compression at relatively low temperature, we are not able to observe that transformation experimentally,’ says Lawrence Livermore’s Federica Coppari. She says work is still ongoing with collaborators to realise these high-pressure phases using different pathways.
Super controversial
Diamond is unusual in its extreme kinetic stability. Coppari and colleagues have observed weird and wonderful bonding behaviour in other compounds, and one of the oddest was first observed in water before being subsequently seen in materials such as methane and ammonia. Following a prediction from researchers at the University of Pennsylvania in 1998, in 2019 Coppari and colleagues used the Omega Laser Facility at the University of Rochester in New York to show that, at pressures above 100GPa and temperatures around 2000K, water enters a ‘superionic’ state. The oxygen atoms remain bonded together into a crystalline lattice, but the protons flow through it freely like a liquid, making the ice electrically conductive through the flow of positive charge.
The description of matter we will have at extreme conditions will be totally different
This has been suggested as a possible source for the magnetic fields of the ice giants Uranus and Neptune, but constraining the properties of high-pressure samples – especially those generated through shock or ramp compression techniques – remains difficult. ‘There are reports saying that the viscosity of this superionic ice may be more like the molten iron inside the Earth’s core, flowing quite rapidly on longer timescales,’ says Coppari. ‘It’s something that would be very interesting to measure but we don’t know how to do it yet.’
Following this, high-pressure physicist Paul Loubeyre of the French Alternative Energies and Atomic Energy Commission and colleagues have conducted static compression experiments on superionic phases of water and other materials using diamond anvil cells. These have revealed, for example, that there are two different superionic structures of water ice that exist at different pressures and temperatures.
One crucial advantage of static compression experiments is that the sample can be cooled at high pressure. This has become especially significant recently as perhaps the most important – and certainly the most controversial – battle in high-pressure research has involved development of room-temperature superconductors. A decade after John Bernal’s thought experiment, a firm prediction that hydrogen would form a metal when the pressure became high enough for the molecules to dissociate was made in 1935 by Eugene Wigner and Hillard Huntington. In 1968, Neil Ashcroft suggested that it might be a high temperature superconductor, and in 2004, he predicted the same for hydrogen-dominated metallic alloys that could be obtained at lower pressures. After much controversy, generally accepted evidence for the molecular metallic state of hydrogen was obtained above 425GPa by Paul Loubeyre’s group in 2020, but the atomic state has not yet been seen.
A race to find ever-higher temperature hydrogen-based superconductors has ensued. In 2014, Eremets and his colleagues at the Max Planck Institute showed that hydrogen sulfide could superconduct at 203K above around 150GPa. Subsequently, multiple groups have produced compounds such as yttrium hydrides and lanthanum hydrides that superconduct at ever-higher temperatures and lower pressures. However, the field has been awash with controversy, with results disputed and papers retracted. The current record is held by LaH10, first documented by Russell Hemley’s group at the University of Illinois, US, which has a transition temperature of 260K, or –13°C, at 200GPa.
Eremets, whose group produced LaH10 independently, says that superconducting metallic hydrogen is the area of high-pressure research he is most excited about in the next few years. Coppari is heavily interested in planetary science, and has in the past published work on Earth’s constituents under core pressure. She is now investigating potential constituents of ‘the planets of the solar system, but also extra-solar planets, which are fascinating because we essentially don’t know anything about those.’ Loubeyre concludes that ‘The description of matter we will have at extreme conditions will be totally different from what we see at ambient conditions. It’s curiosity driven, but the question of how it will affect our lives is unanswered.’
The question of whether or not the same assumptions of chemical bonding are fundamentally useful under extreme conditions is also unanswered. ‘I’m a physicist,’ Pickard says. ‘I follow Schrödinger’s equation, I follow Dirac’s equation, and I look at the consequences – I tend not to spend a lot of time worrying about whether it’s ionic or iono-covalent or whatever, but that’s doesn’t mean it’s not an interesting thing to do.’
Tim Wogan is a science writer based in Oregon, US

Bonds are the ties that bind chemistry

Those seemingly simple sticks belie our most complex concept
- 1
- 2
- 3
 Currently
reading
Currently
reading
When a bond gets too extreme
- 5
- 6
























No comments yet