All non-covalent interactions articles
-
 Research
ResearchStrain can unlock electron-donating orbital in tetravalent carbon atoms
Computational study shows how apical carbons in propellanes and pyramidanes can form hydrogen, halogen, chalcogen, pnictogen and tetrel bonds
-
 Research
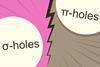
ResearchComputational study says nucleophile finds σ-holes more attractive than π-holes
A σ-hole leads to a stronger bond with NH3 than a π-hole on the same atom
-
 Feature
FeatureReaching into the non-covalent toolbox
Alongside supramolecular stalwarts, budding bonding forms are vying to be valuable, finds Andy Extance
-

-
 Research
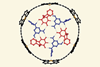
ResearchIntricate supramolecular rosette demonstrates power of cooperative interactions
Rosette assembles inside porphyrin nanoring when all components are present
-
 Research
ResearchModel maps solvent effects on non-covalent interactions
Tool will help experimental chemists pick the best solvent for their reaction
-
 Research
ResearchSerendipitous experiment on xenon complex reveals insights into rare gas interaction
Experiment and theory combo gives benchmark for predicting non-covalent complexes
-
 Research
ResearchMachine learning predicts electron densities with DFT accuracy
Non-covalent interactions and electron densities can be explored quickly without the need for expensive and time-consuming quantum chemical calculations
-
 Research
ResearchStudy ranks interactions between hydrogen-bond acceptors and cations
Experiments establish a set of parameters that will help scientists predict the free energies of the interaction between cations and hydrogen-bond acceptors in any solvent with good reliability
-
 Opinion
OpinionSupramolecular evolution
Can chemists embrace disequilibria like they have non-covalent interactions?