Theoretical work investigating the ability of σ- and π-holes located on the same atom to engage in non-covalent bonds shows that a nucleophile is preferentially attracted to the σ-hole.1
Non-covalent bonds provide the driving force that brings and keeps molecules together. The directional nature of many non-covalent interactions, including hydrogen and halogen bonds, makes them valuable for applications in fields such as crystal engineering and supramolecular chemistry.
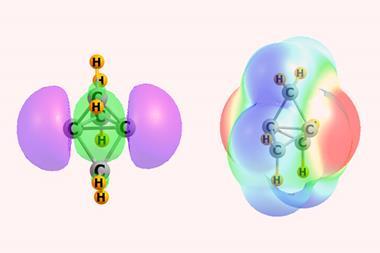
σ- and π-holes are small regions of positive charge within a molecule. For a σ-hole, this region is located along the extension of a covalent bond. For a π-hole, it occurs in the direction perpendicular to the planar framework of a molecule. When these holes interact with a nucleophile, a non-covalent bond is formed. The type of interaction depends on the covalently linked bridging atom – eg a halogen and a noble gas will produce a halogen and an aerogen bond, respectively. Although many molecules contain both types of holes, they are typically located on different atoms, rendering direct comparison difficult.
Previous studies2,3 have looked at molecules containing both σ- and π-holes on the same atom, but have not conclusively answered which type of hole is preferred. Now, Steve Scheiner at Utah State University in the US has used quantum chemical calculations to examine several series of molecules, finding two in which σ- and π-holes occur on the same atom. The first involved T-shaped XR3 compounds, where X is a central halogen atom bearing two lone pairs. The second was T-shaped AeX2Y compounds, where Ae is an aerogen also bearing two lone pairs, X is a halogen and Y is a chalcogen.
In all instances, the σ-hole was deeper and formed a stronger bond with the nucleophile NH3. These findings advance our fundamental knowledge of non-covalent interactions based on σ- and π-holes and their role in controlling crystal structure, paving the way towards crystals with target properties.
References
1 S Scheiner, CrystEngComm, 2025, 27, 921 (DOI: 10.1039/d4ce01194e)
2 W Zierkiewicz, M Michalczyk and S Scheiner, Phys. Chem. Chem. Phys., 2018, 20, 4676 (DOI: 10.1039/c7cp08048d)
3 P R Varadwaj et al, Cryst. Growth Des., 2024, 24, 7789 (DOI: 10.1021/acs.cgd.4c00448)










No comments yet