Strain amplifies tetravalent carbon atoms’ ability to donate electrons, facilitating various non-covalent interactions, quantum chemical calculations show. ‘Usually when a carbon atom is in a tetrahedral bonding situation, which is the most common, there’s no possibility for an electrophile to approach and interact with it. But if you bend back the four bonds that it’s attached to, you can get this to interact, and that has rarely been seen before,’ explains Steve Scheiner at Utah State University in the US, who co-led the study with Wiktor Zierkiewicz from Wrocław University of Science and Technology in Poland.
Carbon atoms donating electrons are not completely unheard of. Some carbenes, for example, can serve as electron donors through their lone pair of electrons. But carbon atoms with four bonds to other species are poor electron donors.
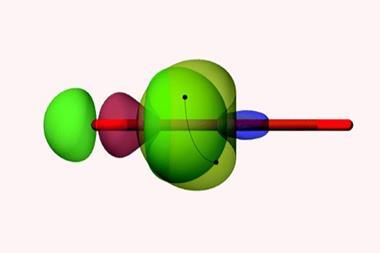
Scheiner and Zierkiewicz, however, have been studying apical carbon atoms in propellane and pyramidane molecules, where the bonding situation is rather different. Along with Mariusz Michalczyk, also at Wrocław University of Science and Technology, they’ve identified an electron-donating orbital – or pseudo lone pair – on these tetrahedral carbons.
While it clearly has a negative charge, Scheiner acknowledges that the nature of this electron-donating orbital could be up for debate. Nonetheless, it appears that this region of negative electrostatic potential can attract the σ-hole of an electrophile to form various non-covalent interactions including hydrogen, halogen, chalcogen, pnictogen and tetrel bonds.
Scheiner says their findings could benefit molecular design: ‘If a chemist, for example, wants to design a system where there’s fairly strong interactions between molecules, inducing some sort of strain into the system with the carbon atom might enable them to ramp up the strength of the interaction with the neighbouring molecule.’
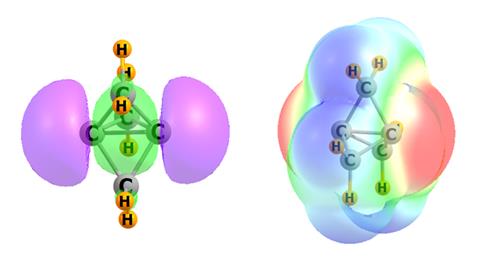
Noting that most organic molecules aren’t as strained as propellanes and pyramidanes, Scheiner wonders how the amount of strain correlates with the interactions they have observed. ‘The next study that I would suggest to anyone is to slowly reduce the strain and see how that changes the effects.’
Goar Sanchez-Sanz, a computational chemist at the University of Manchester, UK, would like to see more on the physical reasoning behind these non-covalent interactions. He highlights that approaches based on absolutely localised molecular orbital energy decomposition analysis, as used in this study, cannot fully elucidate all factors contributing to an interaction. ‘Usually there are a lot of secondary interactions that are, let’s say cloaking, the real one, and dissecting which interaction belongs to what is extremely difficult, sometimes impossible.’
References
This article is open access
M Michalczyk, W Zierkiewicz and S Scheiner, Chem. Sci., 2025, DOI: 10.1039/d5sc01632k








No comments yet