Can chemists embrace disequilibria like they have non-covalent interactions?
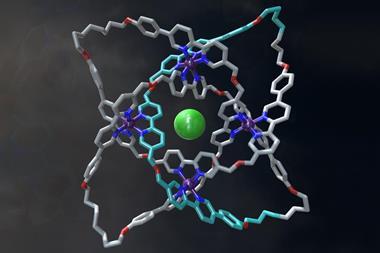
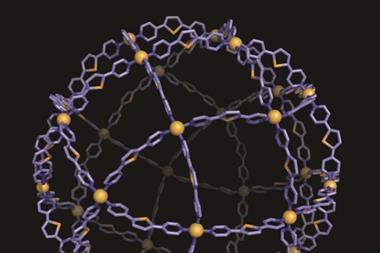
I’m a sucker for a good-looking chemical structure. Chemistry is beautiful in so many ways but you can’t deny the simple satisfaction of a pleasingly aesthetic molecule or complex. The molecular knot and polyhedra we have covered recently are great examples. And when you consider that scientists have designed the basic components of these structures to order and assemble themselves, it makes them even more fascinating.

Chemistry is all about emergent properties. Connecting atoms together to make molecules with altogether different properties is what chemists do day in day out. Supramolecular systems are an extension of the same concept, and as chemists better understand the delicate balance of non-covalent interactions behind molecular recognition they are rationally designing more and more elaborate structures.
Just as empiricism and intuition guided early chemists, gradually developing our knowledge of structure–function relationships, supramolecular chemists are learning about the potential of their structures. Understanding functionality in supramolecular systems is still largely achieved after designing, synthesising and characterising them, but the computational tools that assist in systematically designing and predicting new pharmaceutical chemicals can be applied to supramolecular systems. Just look to biology to see what can be accomplished.
Biology’s achievements in complex self-assembly are humbling. And nature not only makes complex self-assembly look easy, it does it with purpose. If scientists could design synthetic supramolecular systems with just a fraction of the function of those made by nature, the rewards could be huge.
And we’re getting there. It’s only 30 years since Donald Cram, Jean-Marie Lehn (who coined the term ‘supramolecular chemistry’) and Charles Pedersen received the Nobel prize in chemistry ‘for their development and use of molecules with structure-specific interactions of high selectivity’. And last year Jean-Pierre Sauvage, Fraser Stoddart and Ben Feringa shared the prize for developing the idea into rich topologies and chemistries. These chemists have challenged the traditional concept of a bond to make noncovalent and mechanical interactions part of the toolbox, alongside stubborn rigid covalent bonds. But of course biology’s other trick is keeping everything out of the bottom of the potential well.
Most chemical syntheses aim for a stable entity that can be isolated and bottled. Living organisms, however, are dynamic systems – in biology equilibrium is death. So for chemistry to evolve like life, chemists must harness the potential of non-equilibrium systems.










No comments yet