Alongside supramolecular stalwarts, budding bonding forms are vying to be valuable, finds Andy Extance
In 1978, a question that confounded leading chemists of the time drove Gautam Desiraju on a journey that would ultimately lead to an intriguing finding. Desiraju, then a researcher at Eastman Kodak in Rochester, US, was attending the International Conference on the Chemistry of the Organic Solid State (ICCOSS) at Brandeis University in Boston, US. Attendees were all worried about one issue, Desiraju recalls. ‘We didn’t know how molecules crystallise,’ he says. ‘I felt that this was going to be the key problem.’
Desiraju, now at the Indian Institute of Science in Bangalore, soon re-entered academia and sought answers. With his first PhD student he explored how aromatic organic molecules, specifically cinnamic acids, formed crystals. They noticed that adding more halogen atoms to the aromatic rings changed how the molecules packed together, which they called the ‘halogen effect’. Gradually, Desiraju’s team realised that halogen atoms attracted each other, publishing a paper on these halogen–halogen interactions in 1989.
Chemists knew that van der Waals interactions, non-covalent attractive forces arising from fluctuations in electron clouds around atoms, influenced how molecules arrange themselves. From x-ray crystallography data, they knew how closely van der Waals forces made atoms from different molecules pack together. Desiraju and his colleagues proved that distances between halogen atoms were significantly less than expected van der Waals separations. They suspected that this arose ‘because of a certain electrophilic nature of the halogens’, says Desiraju.
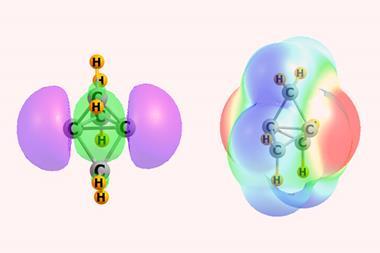
An uneven distribution of electrons around halogen atoms formed electrophilic areas, which have slightly increased positive electric charge. These areas formed attractive interactions with areas of higher negative electric charge elsewhere on other halogen atoms. ‘We found that this effect was more pronounced for iodine, less for bromine, and even less for chlorine,’ Desiraju explains.
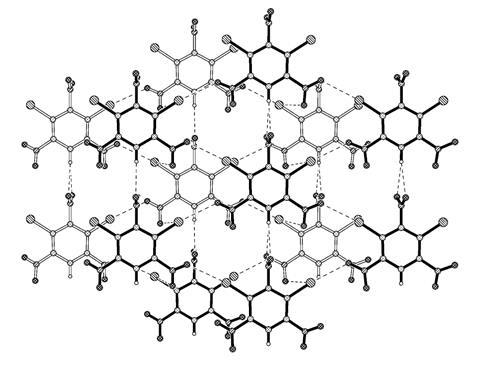
Electrophilic halogens became a key part of the broader concept of halogen bonding, a term first used in 1961. This is somewhat like hydrogen bonding, another common and vital form of non-covalent attraction. In hydrogen bonding, electrophilic hydrogen atoms bonded to electron-withdrawing atoms are attracted to electron-rich atoms like oxygen and nitrogen. In halogen bonding, electrophilic regions of halogen atoms are likewise attracted to electron-rich atoms.
In the last few years, similar concepts have emerged where atoms from group 16 of the periodic table are the electrophile, known as chalcogenide bonds. Analogous interactions exist with group 15 electrophiles, known as pnictogen bonds, and with group 14 atoms, known as tetrel bonds. Another relatively exotic idea is that of weak hydrogen bonding, where the hydrogen atom is relatively weakly electrophilic, because the atom it’s bonded to is less electron-withdrawing, like carbon, for example. But are such bonding interactions any more than a curiosity? ‘Exotic is often different from what is practical,’ Desiraju warns.
Today, these and other recently discovered forms of non-covalent bonding certainly help provide better answers to how molecules crystallise. Desiraju and other scientists can intentionally use them for crystal engineering, with applications including creating pharmaceutical co-crystals that help drug manufacturing. Non-covalent bonding types new and old drive applications spanning the entirety of chemistry, from liquid crystal displays (LCDs) to dynamic medical therapies and sensors for biological processes. Bringing different types of non-covalent bonding together can also create subtle and intricate chemical systems.
Equipping the toolbox
Non-covalent bonding is vital to liquid crystals, like those in the LCD screen you might be reading this on. Such systems mainly rely on van der Waals interactions that, unusually, differ in strength based on direction, explains Duncan Bruce from the University of York, UK. Known as anisotropy, this directionality arises from the shapes of molecules involved, which are typically rigid and either pencil- or disc-shaped. They also contain groups of atoms whose electrons are unevenly distributed, creating partial electric charges known as dipole moments, either permanently or temporarily. Dipole moments can also attract each other.
Together these and other properties modify van der Waals interactions, determining the directionality of a liquid crystal’s structure, which is part way between liquid and solid. They also influence its ability to switch to a different structure in response to a stimulus, such as temperature. ‘There are very many different types of displays with different switching mechanisms and different visual characteristics,’ says Bruce.
Bruce’s team has developed liquid crystals that introduce hydrogen bonding, mixing alkyl-substituted pyridines, specifically stilbazoles, and phenols. ‘Here you’re taking two things, neither of which was a liquid crystal, and then hydrogen bonding them together and making something that was a liquid crystal,’ Bruce explains. And, in 2004, when a colleague showed him a study about halogen bonding, Bruce thought that it might be possible to exploit that too. ‘We could take iodopentafluorobenzene and see if we can make the halogen bonding complex,’ he recalls. ‘And if we could make it, would it be liquid crystal?’ A postdoctoral researcher on his team, Huy Loc Nguyen did some Friday afternoon experiments combining a stilbazole and iodopentafluorobenzene, which was indeed a liquid crystal.
No halogen-bonded liquid crystals have yet been commercialised because they lack long-term stability, Bruce says. Yet he stresses halogen bonding’s importance as one part of ‘a toolbox of synthetic methods and interactions’ available to chemists, he adds. ‘The creation and use of the toolbox is the work of many talented and imaginative people. Non-covalent interactions are fundamental to that toolbox. When you have a new means of doing something, you bring new people to the field and that is always positive as it refreshes thinking and challenges existing orthodoxies. It also sparks imagination in chemical design, which can then spin off in so many other directions.’
Since 2004, Bruce has also studied halogen-bonded liquid crystals with the teams of Pierangelo Metrangolo and Giuseppe Resnati at the Polytechnic University of Milan in Italy, who are pioneers in halogen bonding research. Metrangolo notes that the first report of such a bond was published in 1863 by Frederick Guthrie from the Royal College in Mauritius. Yet nobody intensively studied halogen bonds until the 1990s. Metrangolo says that he and his colleagues have convinced people that they can be ‘as effective as hydrogen bonds, and sometimes even better’ in fields as diverse as liquid crystals, crystal engineering, polymers and ion sensing.
Metrangolo believes that the most important recent findings his team has made concerning halogen bonding involve biological molecules such as amino acids and proteins. Specifically, they concern the toxic process known as oxidative stress thought to be involved in many diseases. In the best-known oxidative stress pathways, peroxides produce free radicals that cause widespread damage to cells. Metrangolo says that in the next most common oxidative stress pathway, halogens can react with and damage amino acids in proteins. ‘We have had many results showing that proteins can be misfolded upon adding some halogens into the structure of some amino acids,’ he explains. The newly added halogen atoms are responsible for attractive non-covalent bonding causing the misfolding. This helps understand issues like cystic fibrosis, sepsis and skin ageing, Metrangolo adds.
Dynamic detection
Anthony Davis’s team at the University of Bristol in the UK reaches deep into the non-covalent bonding toolbox to make chemical systems that recognise carbohydrate molecules. They can help in technology that recognises glucose sugar molecules to manage and treat diabetes. Davis highlights several other attractive interactions his team might make use of, including electrostatic interactions between molecules carrying opposite electronic charges.
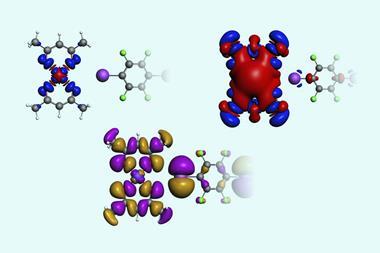
Davis often relies on clouds of electrons surrounding aromatic rings originating from double bonds between carbon atoms, known as π electrons. Such molecules have a ring of negative electric charge directly around carbon atoms, surrounding a central positive charge. Stacked rings can be offset, so that the positive charge is located above a negative charge on the ring below, forming an attractive interaction. Alternatively, the clouds of electrons can attract cations or electrophilic hydrogen atoms attached to other atoms, such as oxygen or carbon atoms. Electron-rich π systems can also stack alternately with electron-poor π systems, which is referred to as a π-donor-acceptor interaction. Perhaps surprisingly, even hydrogen atoms attached to carbon atoms can form attractive CH–π interactions.
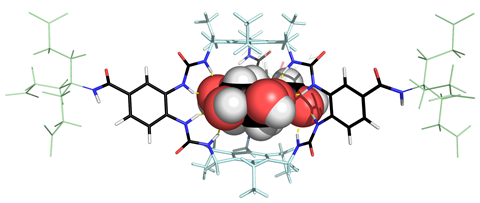
‘Carbohydrates have got a lot of CHs and we have always tried to place π surfaces against them, and it’s tended to work,’ says Davis. ‘It will be stronger if the hydrogen is electron deficient and the oxygens in glucose presumably help in this respect. It is also more noticeable in water because it is supported by the hydrophobic effect as neither CH nor π surfaces are fond of water.’ To get the best recognition, the Bristol team tries to make supramolecular systems combine different non-covalent interactions that complement the target they want to bind. ‘We’d be looking for hydrogen bonding and nonpolar interactions, but CH–π interactions are particularly good.’
Danish pharmaceutical company Novo Nordisk is using Davis’s team’s glucose recognition technology to develop adaptive insulin molecules. These agents could circulate in the body of a person with diabetes, activating themselves when needed, rather than them requiring regular insulin injections. ‘You have insulin with a receptor at one end and the glucose unit at the other,’ explains Davis. In blood low in glucose, the two ends of the molecule come together, inactivating it. But when glucose levels rise, a free sugar molecule can displace the tethered one. In this conformation, the insulin can tell the body to lower glucose levels. ‘You produce insulin which is active when you want it to be active,’ Davis says.
Nature knows about non-covalent interactions much better than us
Claudia Caltagirone’s team at the University of Cagliari in Italy likewise develops chemical recognition systems, which contain fluorophores or chromophores that change colour or emit light when they bind ions. Including these light signals lets the Cagliari researchers detect very low ion concentrations, down to nanomolar levels, using optical cameras. They could work in real time, directly in the environment, Caltagirone explains. Her team is also working on novel soft supramolecular materials, in which the building blocks can self-assemble via non-covalent interactions, which can trap pollutants to help clear up contaminated environmental sites.
Metal cation recognition involves classic covalent coordination chemistry. But when Caltagirone’s team wants to capture anions of many sizes and shapes, for example environmental pollutants such as nitrate and phosphate, they reach for the non-covalent toolkit. ‘We can have hydrogen bond formation, halogen bond formation, CH–anion, π–π stacking, and π–anion interactions,’ Caltagirone says. ‘In our lab, we normally design neutral receptor systems that interact with anions via hydrogen bonds.’ However, as one example of a different interaction, in a pyrophosphate anion detection system, their fluorophore was a naphthalene with a CH well positioned to bind the anion. Beyond such tools, Caltagirone points to nature for evidence that exotic forms of non-covalent bonding can be important.
Halogen bonding is essential to the thyroid hormones thyroxine and triiodothyronine, which work ‘only because there is iodine in there’, Caltagirone stresses. Likewise, the enzyme glutathione peroxidase only works because it has a selenium atom that forms non-covalent chalcogen bonds. ‘Nature knows about non-covalent interactions very well, probably much better than us,’ Caltagirone underlines. ‘For this reason, it is worth keeping on studying them.’
Such studies might enable researchers to discover further unusual non-covalent bonds, like the platinum–platinum interactions studied by Vivian Wing Wah Yam at the University of Hong Kong.
Platinum shines
Yam became interested in interactions between platinum atoms after spending two visiting fellowships with Geoffrey Wilkinson at Imperial College London, UK, in 1991 and 1992. She was working on luminescent metal coordination complexes but felt limited by existing structures. Their colour originated because they absorbed light, making electrons move from the metal atoms at the complexes’ centre to ligands surrounding them. Usually such complexes relied on carbonyl ligands, which left chemists with fewer options to alter. Exploring alternative ligands, Yam found she could make platinum(II) and gold(III) complexes phosphorescent in solution, she tells Chemistry World.

Researchers initially discovered that there could be non-covalent bonding interactions between platinum atoms from solid square-planar platinum(II) complexes, Yam explains. Such complexes could exist in different coloured forms, for example red or yellow, and initially the difference wasn’t clear. But then x-ray crystallography showed that platinum atoms in the red form are much closer to each other. Studies eventually showed that d- and p-orbitals from each atom overlap and mix, forming non-covalent bonding interactions that ultimately stabilise the structure that brings platinum atoms nearer to each other.
This could be much more versatile for tuning luminescence colours, Yam realised. ‘It’s a flat molecule, you can now start to stack them and play around with supramolecular assembly,’ she says. As one example, one platinum complex with bis(benzimidazolyl)pyridine ligands self-assembles to produce a magenta-coloured solution in water. In a mixture of 80% acetone in water, the solution is blue. In water they mainly assemble due to hydrophobic interactions, with a loose platinum–platinum interaction providing the magenta colour. In the acetone/water mixture, they assemble through tight platinum–platinum interactions turning the solution blue.
In 20 years of working on such systems, Yam’s team has developed many uses of non-covalent platinum–platinum interactions. The Hong Kong researchers have used the complexes’ luminescent qualities in organic light emitting diodes. They have also patented solution-phase sensors that change colour in the presence of molecules such as RNA or DNA. None of the potential applications that Yam’s team has explored has yet been commercialised, but she thinks that sensing is most likely to be practically useful.
Yam’s team has also taken donor–acceptor interactions from the non-covalent toolbox to help control how their platinum systems assemble. The pyridine ligands that the Hong Kong researchers use stack up one on top of the other due to platinum–platinum interactions with partial π-π stacking. Each layer faces the opposite direction to those above and below, in a head-to-tail configuration, says Yam. Modifying the ligands around the platinum atoms to incorporate donor–acceptor interactions ensures all the layers align in the same direction. The difference between the strength of the platinum–platinum non-covalent bonding and the electron donor–acceptor interaction completely changes the mechanism through which the system assembles too, explains Yam.
Cocrystal creation
In the solid phase, non-covalent interactions have been making an impact on the pharmaceutical industry. Desiraju and other researchers have developed ways to predict the structures that molecules will form when they crystallise, answering the question posed at ICCOSS. Desiraju developed a technique known as the synthon approach, identifying building block structures that molecules come together to form before assembling as a large overall crystal. For example, simple aromatic carboxylic acids will pair up to form simple hydrogen-bonded dimers 70–80% of the time. Loading more functional groups onto the molecules brings together different interactions that create preferred patterns. Such knowledge enables scientists formulating drugs in the pharmaceutical industry to design crystals that incorporate ingredients specifically intended to help their products dissolve and travel through patients’ bodies. Today fewer than 10 drugs have used such capabilities, but it has the potential to be a ‘really big practical application’, Desiraju says.
People want to find new interactions. The future will tell whether these have an impact or not
Most interesting of all, for Desiraju, is the potential to bring together three or four molecules in a single cocrystal for each of their properties. But creating a crystal comprising building blocks containing one of each of the molecules is surprisingly difficult, Desiraju explains. ‘Suppose I have four molecules ABCD, and suppose interactions of the type A to B, B to C and C to D, are all strong,’ he says. ‘You will just get binaries AB, BC and CD.’ To get more molecules to come together as ternary or quaternary crystals requires non-covalent bonds that are graded in strength. For a ternary compound ABC, A and B could experience the strongest interaction, like conventional strong hydrogen bonds. B and C could experience the second strongest interaction, which might be a halogen bond. Finally, the attraction between C and A could be weakest, such as a weak hydrogen bond. A could have medicinal properties, B could boost solubility, and C could help permeability, Desiraju suggests.
Cocrystals also provide a specific example of how halogen bonding can be useful, Metrangolo adds. He highlights the molecule iodopropynyl butylcarbamate, which is often used a preservative in cosmetics, paints and coatings. Its melting point is relatively low, around 66°C, which makes it very sticky and hard for manufacturers to use. The iodine atom in the molecule is very electron poor, meaning that it can halogen bond with chlorine atoms in calcium chloride. Metrangolo, Resnati and colleagues have patented the resulting cocrystal of the two, which melts at around 82°C and is therefore much easier to handle. Metrangolo’s team is now working to develop co-crystals of halogen-based chemotherapy drugs used to treat cancer, to make them soluble in water as opposed to dimethylsulfoxide, their current solvent. ‘Halogen bonding for improving the properties of pharmaceutical compounds is still under-explored,’ he says.
With so many different types of non-covalent bonding possible, some scientists are looking to find a way to organise them, Metrangolo adds. ‘It is nowadays very well accepted that the interactions are a property of the atoms,’ he says. ‘People are speaking of a periodic table of interactions.’ Making their strengths and weaknesses obvious could be important, because Metrangolo is uncertain that every non-covalent bonding interaction will prove useful. ‘People want to find new interactions,’ he says. ‘The future will tell whether these have an impact or not.’
Yet even when the application of a non-covalent bonding interaction is unclear, we should have patience, says Davis. One member of his team, Tiddo Mooibroek, is now actively exploring an exotic non-covalent bonding interaction. He’s looking at tetrel bonding involving carbon atoms in the solvent tetrahydrofuran and 3,3-dimethyl-tetracyanocyclopropane. This work reminds him of when he first read about halogen bonding decades ago. Davis did rather think ‘How is anyone ever going to use this?’ he explains. ‘It’s beginning to look like it will be rather useful, particularly in the area of anion binding and anion transport across cell membranes. That could have a variety of useful effects, maybe antibiotics, maybe anti-cancer, or cystic fibrosis, where natural anion transport isn’t functioning properly. In that area, halogen bonding does look like it might be really rather useful. The main message is don’t write anything off in the early stages.’
Andy Extance is a science writer based in Exeter, UK
Bonds are the ties that bind chemistry

Those seemingly simple sticks belie our most complex concept
- 1
 Currently
reading
Currently
reading
Reaching into the non-covalent toolbox
- 3
- 4
- 5
- 6























No comments yet