All stereoselective articles
-
 Research
ResearchBoron building block assembles tetra-substituted alkenes like Lego
Powerful method offers control of stereochemistry to produce drugs and natural products
-
 Research
ResearchEnzyme-inspired ligand distinguishes between simple alkyl groups
Designer ligand creates active site-like pocket for asymmetric radical reaction
-
 Research
Research‘Transformative’ carbonyl cross-metathesis used to synthesise alkenes
Two new catalytic methods can produce a broad range of Z- and E-alkenes with remarkable cross-selectivity, paving the way for more efficient organic syntheses
-
 Research
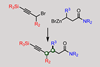
ResearchNickel catalyst switches on double stereochemistry in reaction between racemic alkanes
First-of-its-kind reaction creates stereocentres by stitching together racemic nucleophiles and electrophiles
-
 Opinion
OpinionStereoselectivity with a twist
Helical ligands amplify solvent chirality to control reaction outcomes
-
 Research
ResearchNew tools direct reactions at specific C–H bonds in organic molecules
Elegant catalysts offer quicker routes to complex compounds
-
 Research
ResearchChallenging mirror molecules made with stereochemistry destroying reaction
Clever catalyst repurposes classic nucleophilic substitution to make chiral quaternary carbon centres
-
 Research
ResearchCatalyst sets sights on C–H sites
New catalyst stereoselectively functionalises unactivated C–H bonds
-
 Research
ResearchRoute to complex amines holds drug development promise
New catalytic reaction yields products with three adjacent chiral centres
-