A new catalyst promotes an asymmetric radical reaction using an engineered ligand that can differentiate between very similar alkyl groups. The stereoselective amination process forms highly enantioenriched α-chiral alkyl amines, a common motif in drug molecules.
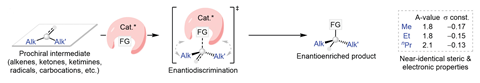
Radicals are a common intermediate in many synthetic transformations. Their single electron makes these species extremely reactive, enabling rapid reactions with minimal energy input. However, for prochiral substrates – those with three different groups attached to the radical centre – controlling the enantioselectivity of the subsequent mechanistic steps is an almost impossible challenge.
‘The main difficulties arise from the subtle structural differences between the alkyl groups, the tendency for non-stereoselective background reactions to dominate and the extremely low energy barriers that make it hard to control enantioselectivity,’ explains Xin-Yuan Liu, who researches radical chemistry at the Southern University of Science and Technology in China. Despite these issues, prochiral di-alkyl radicals are still some of the most widely generated radical intermediates in synthetic chemistry, making the lack of stereocontrol an ongoing frustration for organic chemists.
Taking inspiration from enzymes’ ability to differentiate between similar alkyl substituents within the active site, Liu’s team sought to address this selectivity problem with a carefully engineered metal–ligand complex that incorporates a reactive pocket to pre-organise the troublesome substrates. By holding the prochiral radical in a single reactive conformation within the cone-shaped hole, they hypothesised that subsequent reaction steps would occur on the same side of the molecule, thereby giving a single-enantiomer product.
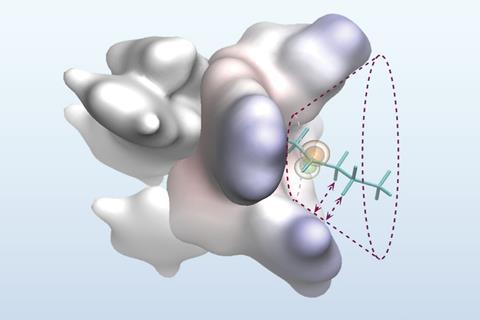
‘During the reaction, smaller parts of the molecule fit into the tight inner area, while larger parts are pushed toward the roomier outer region,’ explains Liu. ‘Special bulky features placed around the edge of the pocket help guide the reaction by forming gentle, non-bonding interactions – like weak attractions between hydrogen atoms and nearby ring systems.’
The team focused on developing an asymmetric amination reaction, using established copper chemistry to stereoselectively transfer a sulfoximine group onto various di-alkyl radicals to form α-chiral alkyl amines. The optimised reaction, which accommodated both thermal and photocatalytic radical processes, tolerated a variety of functional groups and effectively distinguished between similar alkyl and cycloalkyl substituents, forming more than 50 different chiral amines in up to 96% enantiomeric excess.
‘The scope of this chemistry mainly features prochiral radicals bearing methyl substituents, which maximise the chance of structural differentiation within the radical intermediate,’ explains Mattia Silvi, an organic chemist at the University of Nottingham in the UK. ‘However, substantial differentiation between an ethyl and a propyl group is observed in one of the entries. I found this entry particularly interesting and testament of the remarkable potential of this asymmetric strategy.’ Silvi was further impressed by the team’s rigorous catalyst design and believes this enzyme-inspired approach will stimulate others in the field to develop similar strategies for different reactions.
Meanwhile, Liu’s team is already investigating how this methodology could be extended to a wider range of transformations, including forming carbon–carbon and carbon–heteroatom bonds. ‘In addition, we’re working on developing asymmetric reactions involving unactivated prochiral trialkyl-substituted alkyl radicals – a particularly challenging but promising direction that could open up even more possibilities in chemical synthesis,’ Liu says.
References
YF Zhang et al, Science, 2025, DOI: 10.1126/science.adu3996














No comments yet