First-of-its-kind reaction creates stereocentres by stitching together racemic nucleophiles and electrophiles
Researchers have developed a reaction that has long been on many chemists’ wish lists: joining up racemic alkanes to create a compound with two adjacent stereocentres in a single step. Experts describe this first-of-its-kind reaction as a powerful strategy for making particularly challenging carbon–carbon bonds.
The reason alkane coupling is on the list of dream reactions is that it allows chemists to add a third dimension to their molecules. One of the most used reactions in medicinal chemistry, the palladium-catalysed cross coupling, joins up aromatic or other sp2 carbon atoms. For alkyl–alkyl couplings, however, chemists usually stick with classic nucleophilic substitutions. But these are limited to a small number of nucleophiles and electrophiles, are prone to side reactions like eliminations and usually hopeless when it comes to creating chiral centres.
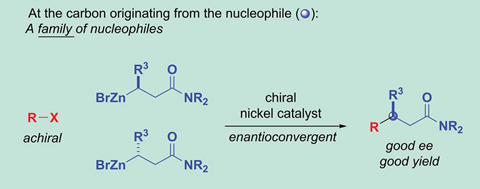
Gregory Fu and his team from the California Institute of Technology, US, have developed a nickel catalyst that bonds alkyl nucleophile and alkyl electrophiles – both secondary, racemic carbon compounds – to make a single stereoisomer with two chiral centres. The reaction uses propargylic halide electrophiles and β-zincated amide nucleophiles. Enantiomeric excess is around 90% in all 30 examples Fu’s group describes, with diastereomeric ratios close to 99:1 in most cases.
‘When you think about disconnecting molecules, you want to clear stereocentres and make carbon–carbon bonds,’ says Mary Watson, an organic chemist from the University of Delaware, US, who wrote a perspective piece about the study but otherwise wasn’t involved. ‘This process lets you do that all at once and I think that’s quite powerful.’
Although the coupling partners need to carry certain functional groups – an alkyne and an amide, respectively, ‘I think this is a reaction people will absolutely use’, Watson says. In fact, both groups can be converted into a number of useful functionalities.
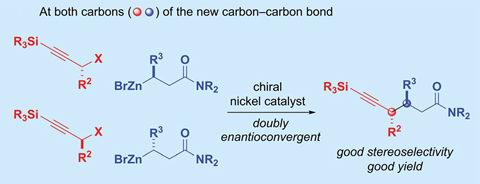
Fu says that this is the first reaction of its type. Previous enantioconvergent couplings – those that convert a stereochemical mixture into a single stereoisomer – could only deal with one racemic coupling partner; the other one needed to be achiral.
All stereochemical control comes from the catalyst, which carries a chiral pyridine-oxazoline ligand. ‘The new C–C bonds are forged by overriding the inherent chiral information of both starting materials and bringing them together selectively to construct two adjacent – vicinal – stereocentres’, comments Ohio State University chemist David Nagib, who calls the study ‘exciting’. ‘Many will be eager to apply this method,’ he says.
Fu and his team are now working on accessing the other enantiomeric pair the reaction theoretically could produce as well as broadening the scope of nucleophiles and electrophiles.
References
H Huo, B J Gorsline and G C Fu, Science, 2020, 367, 559 (DOI: 10.1126/science.aaz3855)












No comments yet