New catalytic reaction yields products with three adjacent chiral centres
A new method allows chemists to obtain highly complex amines with up to three adjacent stereocentres. The process is highly selective and has great potential in drug development, where having enatiomerically pure products is important for evaluating their properties.
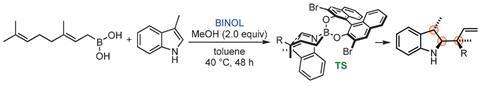
Researchers led by Kálmán Szabó at Stockholm University in Sweden came up with a clever divergent synthesis that allows them to obtain all the different possible products a la carte. The approach uses allylboration catalysed by chiral naphthols to functionalise both indoles and cyclic imines with terpene-based moieties.
To obtain a product with three adjacent stereocentres, Szabó and his team used skatole (3-methylindole) as a starting material. They were able to control the outcome of the reaction by modifying the catalyst, the stereochemistry of the boron derivative or both. Using all the different combinations, they can selectively obtain the four possible products with excellent enantiomeric purity.
Because indoles, cyclic amines and terpenes are common structures in many natural products and drugs, the authors envision many applications for their new methodology in the field of medicinal chemistry.
References
R Alam et al, Angew. Chem., Int. Ed., 2016, DOI: 10.1002/anie.201608605
















No comments yet