The first superatoms made using actinides have been created, continuing to upend our understanding of how f-block elements form bonds and adding a new dimension to one of the strangest chemical phenomena.
Superatoms are unusual structures first discovered 40 years ago, where clusters of atoms exhibit the properties of elemental atoms due to quantum confinement effects. This can result in radically different behaviour than would otherwise be expected; for example, a cluster of Al13 acts in a similar manner to elemental chlorine. This unique shifting of how the clusters react has meant superatoms can be used as nanoscale building blocks, with chemists able to fine-tune the properties of structures as required.
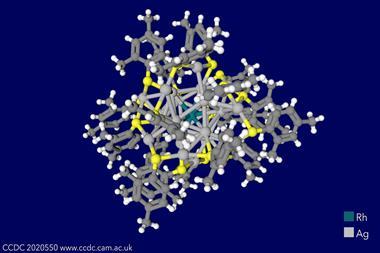
Superatoms gain these strange properties thanks to metal–metal bonds. While previous superatoms have contained actinides, they have only been embedded in superatoms made up of clusters of main group or transition metal elements. Now, a team led by Stephen Liddle at the University of Manchester, UK, has created a superatom that features the actinide thorium.
Actinides are difficult to work with. They are radioactive and compounds are often thermally unstable, with powdered samples decomposing in days and solutions rapidly decomposing at over 35°C. Previously, the group created a σ-aromatic thorium, the first aromatic ring made solely from actinides and the first time an actinide–actinide bond had been created. This earlier work overturned previous predictions, which suggested that actinide–actinide bonds would be weak, rather than the strong bonds typically seen in delocalised aromatic structures.
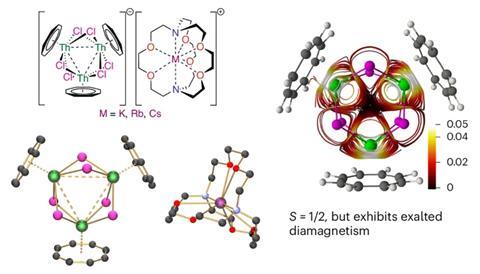
This new paper pushes the boundaries of actinide chemistry even further. The superatom structure is based around a trithorium nanocluster – three thorium atoms forming an aromatic ring – supported by a scaffold of chlorine, with each thorium atom also supporting a wing-like aromatic ring.
While this provides further evidence for strong actinide–actinide bonds, the molecule also comes with a ‘mind-blowing’ twist, Liddle says. ‘Although [the electron structure is] unambiguously paramagnetic, [the molecule’s] magnetism is diamagnetic.’
Diamagnetism is where materials are repelled by magnetic fields, rather than attracted; the exact opposite of what would be expected for an actinide. Although diamagnetic molecules are common (the best example is water), the result indicates that the actinide bonds are causing ‘exalted’ diamagnetism – greater than expected diamagnetism due to an aromatic ring. This flip in expectations shows the structure is a superatom; while both group 1 and group 11 elements show diamagnetism, as it has one electron cluster, the team determined that the superatom is behaving like an element from group 1 of the periodic table, or as a ‘super alkali-metal’.
This latest paper is an ‘important expansion’ of the team’s earlier work, says Thomas Albrecht, the director of the Nuclear Science and Engineering Center at the Colorado School of Mines, US. The original paper ‘resulted in quite a bit of controversy’, Albrecht adds, ‘but their explanation has stood the test of time thus far. The observation of diamagnetism is the most remarkable aspect of this cluster. The superatom behaviour, and rather counterintuitive aromaticity that gives rise to it, is really not what most people would think of. It’s an impressive study.’
References
JA Seed et al, Nat. Chem., 2025, DOI: 10.1038/s41557-025-01790-3












No comments yet