Just a few years ago, predicting how a string of amino acids assembled into a functional protein was one of biology’s most formidable puzzles. That was until artificial intelligence (AI) cracked the problem wide open, earning global acclaim and even last year’s Nobel prize in chemistry.
That breakthrough relied on deep learning, a form of AI that learns from massive datasets to make predictions, such as the shape of proteins. Now, researchers are building on that momentum with generative AI. A powerful new class of AI model that, rather than just predicting structure, can imagine entirely new possibilities – from unknown protein sequences to novel treatments for a wide range of diseases.
‘Most protein design work starts with a perfect, experimentally determined 3D map of the target,’ says Timothy Jenkins of the Technical University of Denmark. ‘But for many of the most important [therapeutic] targets … these maps simply don’t exist. This has been a major roadblock for personalised medicine.’
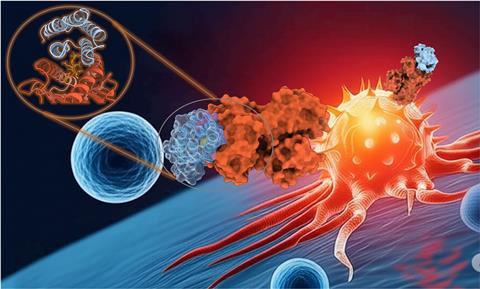
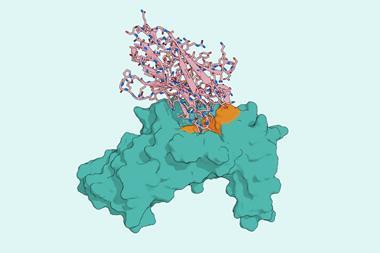
Jenkins and his collaborators recently published a study in which they used a generative AI model called RFdiffusion to design molecules that help the immune system recognise and attack cancer.1 ‘Put simply, we created a new, ultra-fast pipeline for making precision cancer immunotherapies,’ he says.
Game changer for targeted therapies
The process works like a high-speed assembly line, with different AI tools handling different steps. ‘First we show RFdiffusion a 3D picture of the cancer flag we want to target,’ explains Jenkins. This 3D picture can come from experimental data or from AI-based structure prediction. ‘The AI then “dreams up” thousands of completely new, small protein shapes … that it thinks will fit perfectly onto that target,’ he adds.
A second AI model takes those shapes and figures out the exact amino acid sequences needed to build them. ‘This process is incredibly fast,’ says Jenkins. ‘Generating thousands of potential designs on a computer takes a matter of hours or days, whereas finding just one candidate using traditional lab methods can take months or even years.’
Molecular dynamics simulations are then used as a ‘virtual crash test’ to see how well the designed proteins stick to their cancer targets, helping the team narrow down candidates before moving to lab experiments.
‘The fact that we were able to create a successful binder based on a purely computational prediction is a game-changer,’ says Jenkins. ‘It demonstrates that our approach is not limited to the small number of well-characterised targets and can be extended to design therapies for truly personalised cancer targets, for which no structural information is available.’
Dreaming up proteins to kill deadly bacteria
Beyond personalised cancer therapy, a group led by Rhys Grinter at the University of Melbourne and Gavin Knott at Monash University are using generative AI to design proteins that kill antibiotic-resistant bacteria.2
‘AI-based protein design effectively enabled us to dream small proteins into existence that would bind ChuA,’ says Grinter. ChuA is an outer membrane transporter protein used by pathogenic bacteria Escherichia coli and Shigella to extract haem – a rich source of iron – from their hosts.
Using AlphaFold2, the team predicted the 3D structure of ChuA from its amino acid sequence in minutes. The model was then evaluated for accuracy, and a strategy was developed to block ChuA’s function. Generative AI tools, RFdiffusion and ProteinMPNN, were subsequently used to design proteins capable of interfering with the target.
‘The entire process took a few weeks, with a success rate of 10–50% for functional designs,’ says Knott. ‘This approach effectively shaves months to years off the standard experimental structural biology approach and rapidly accelerates development of novel biologics.’
‘It’s amazing and underscores the power and transformative potential of AI in protein design,’ adds Grinter.
Grinter, Knott, Jenkins and their colleagues favour AI platforms that are freely accessible to the wider scientific community. Making these tools available to all has helped lower barriers for researchers worldwide. As a result, a wider range of experts can collaborate and innovate, accelerating progress on pressing societal challenges such as antibiotic resistance and personalised cancer therapies.
‘However, it’s not as simple as point and click,’ says Knott. ‘At the moment, the technology still requires a deep understanding of protein structure and function relationships.’
And there is still a need for caution because, despite the impressive capabilities of generative AI, it has inherent limitations rooted in the data it learns from. ‘Generative AI is incredibly powerful, but it’s not magic,’ says Jenkins. ‘Models are trained on the thousands of protein structures we already know. If we ask them to design something radically different from anything they’ve “seen” before, they can sometimes “hallucinate” a design that looks good on the computer but isn’t physically stable or functional in the real world.’
‘Biology exists in the real world, so AI achieves real value when coupled with experimental techniques and applied to biological systems,’ concludes Grinter. ‘Combining experiments and AI has transformative power for scientific discovery and technology development. We think in the future that AI tools will form a central component of almost all aspects of biological research.’
References
1 K H Johansen et al, Science, 2025, 389, 380 (DOI: 10.1126/science.adv0422)
2 D R Fox et al, Nat. Commun., 2025, 16, 6066 (DOI: 10.1038/s41467-025-60612-9)











No comments yet