Turing patterns show their hand in finger formation
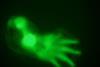
After 62 years, scientists clinch the identification of molecules that confirm codebreaker’s ideas in a different ‘digital’ area

After 62 years, scientists clinch the identification of molecules that confirm codebreaker’s ideas in a different ‘digital’ area