New materials created in printed plastic vessels that can withstand high temperatures
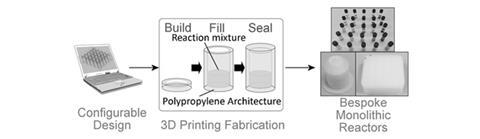
Performing hot chemistry in plastic reaction vessels may sound perverse, but UK researchers have shown that it is possible to carry out a range of hydrothermal chemical syntheses in sealed reactors made from 3D printed polypropylene.
The approach enables sealed lab-scale reactors of, for example, 1ml or 2 ml capacity to be printed, with the possibility to scale up to bigger vessels as required. It also allows the printing of grids of sealed chambers – in the new work a 5x5 array was fabricated – for high-throughput screening of reactants and conditions. Normally, for the type of reaction under (hydrothermal synthesis involving heating mixtures at up to 140°C for many hours) expensive precision-machined stainless steel autoclave bombs are usually needed. The new work could make such experiments far less costly and more accessible to less well-resourced labs.
The work was led by Lee Cronin and Ross Forgan at the University of Glasgow, who had already pioneered the use of 3D printing for creating reactionware of specific geometrical configurations to help control the chemistry within. ‘I wanted to know if we could extend the idea of using 3D printing to make compartments that could tolerate heat,’ says Cronin. ‘What about solvothermal chemistry?’
Using polypropylene, the team first partly printed the vessels. ‘We then pause it in the middle, add the reagents, finish the printing so that the reagents are sealed inside, and put it in the oven,’ Cronin says. After heating at an appropriate temperature and time, the vessel is removed, drilled or cut open and the products removed.
If the reactors were heated to 150°C, the plastic deformed and the vessels failed. But below this temperature the vessels retained their integrity. To test whether the vessels could successfully host solvothermal chemistry, the group synthesised two well characterised metal-organic frameworks (MOFs); the first in a 3 ml capacity reactor that required heating for 18 hours at 130°C, and the second in a 20 ml reactor that was heated at 140°C for 18 hours. In both cases, the yields and sample purity were comparable with conventional methods.
To test the system’s versatility, the researchers printed a 5x5 grid of reaction chambers with a 1ml capacity. In a bid to create novel co-ordination polymers, each chamber was charged with a combination of diacid linkers, bis(pryidine) based pillars and metal cations. After heating at 120°C for 48 hours the array was removed and holes drilled into each chamber to identify ‘hits’ – wells that contained crystals suitable for structure studies. In this way, and following further experiments with the reactor array to identify optimal synthetic conditions, two new three-dimensional coordination polymers were discovered. A larger printed reactor was then used to scale up the synthesis of one of the polymers, providing a sample comparable to that prepared with conventional equipment.
‘We have shown that the concept works, and that you can use it to discover new materials,’ says Cronin. ‘Clearly there are limitations involving solvent compatibility and taking the temperature beyond 150°C. But most hydrothermal reactions use alcohol or water and most are done at 140 to 150 °C. Also there are other plastics available that do melt at higher temperatures.’
‘Cronin and Forgan’s work has enormous potential in the development of new materials,’ says Neil Champness, head of inorganic and materials chemistry at the University of Nottingham in the UK. ‘One of the key results is the ability to explore a large a variety of reaction conditions in a relatively simple and cheap manner. This should accelerate materials discovery in this field of research and for the area of MOFs where there are almost limitless combinations of components this is an enormous step forward.’











No comments yet