Companies have put biofuels on the back burner to aim for higher margin chemicals, as Emma Davies finds out
Companies have put biofuels on the back burner to aim for higher margin chemicals, as Emma Davies finds out
Tom Welton gives the wooden desk in his office a sharp rap with his knuckles. ‘That’s the sound of lignin,’ he says, grinning. ‘Have you seen its structure? It’s beautiful, full of aromatics, lovely compounds that make you think: I could make something useful from this.’
Welton, who is head of chemistry at Imperial College London, UK, views lignin – the ‘really hard stuff’ that protects plants from biological attack – as a valuable source of renewable speciality chemicals. His group has found a way to extract it from lignocellulosic biomass using ionic liquids and has plans to make polymers using the technology.
In industrial biochemical and biofuel production, lignin is commonly viewed as little more than waste, sometimes burned to generate energy. Instead, the focus is on biomass cellulose, a polymer composed of repeated units of glucose, locked deep within the complex matrix of lignin and polysaccharides that make up lignocellulose.
Lignocellulosic biomass has long been touted as the biofuel redeemer, after first generation biofuels received a bad press for taking over fertile farmland needed for food crops. Yet economic pressures and technological challenges are steering many companies away from fuels, towards renewable chemicals production from lignocellulosics.
The biofuel bubble has not burst, but it was deformed by the fact that oil prices have not risen as quickly as predicted several years ago. Fuels are a very high-volume, narrow-margin business and volume targets are hard to meet without making enormous investments in new biorefineries. ‘Five years ago, access to loans for construction of new biorefineries basically dried up,’ says Mike Knauf, chief executive of renewable chemicals company Rivertop Renewables in Montana, US.
‘Left in that situation, what did all these great biotechnology companies do?’ he asks. ‘They said: let’s redirect to find valuable markets outside of fuels; let’s scale down so we don’t need as much capital investment and let’s see what we can do. And one after another they found niches. It doesn’t mean that biofuels can’t happen and won’t happen – it means the timing wasn’t right,’ Knauf adds.
In some cases, renewables start-ups are opting for higher-margin products such as speciality chemicals to help them through the early days of make-or-break, says Mark Mascal, professor of chemistry at the University of California in Davis, US, who is also involved in commercial bio-based chemicals production.
Field of dreams
Of course, producing chemicals from plants is not new. In 1941, US company Ford produced a concept car with a body made partially from products derived from hemp and soya beans. Henry Ford is reported to have asked ‘Why use up the forests, which were centuries in the making, and the mines, which required ages to lay down, if we can get the equivalent of forest and mineral products in the annual growth of the fields?’
The second world war put paid to Ford’s dream, but the concept of creating chemicals from crops is once more taking hold and there is a growing commercial market for renewable chemicals. Rivertop is using nitric acid to oxidise sugar to create glucaric acid, aimed initially at the detergent market. ‘It’s a classic nitric acid oxidation that has been around for more than a century, but what we have done is put some important new developments in, including the recycling of the nitric acid. We are able to oxidise the byproducts to regenerate nitric acid, using the acid as a catalyst rather than a consumable,’ explains Knauf.
A chemical process has a lot of advantages over using microorganisms
The company has done small-scale pilot tests and is getting ready to scale up. Its process currently uses purified glucose from starch, which is ‘readily available and inexpensive,’ says Knauf. ‘Long term, we see ourselves going for the lowest cost and the most renewable, sustainable sources of sugar, which could be lignocellulosic biomass,’ he adds. ‘One of the interesting things about lignocellulosic biomass is there are all sorts of sugars in there other than glucose. I find that very interesting; being able to work with xylose, galactose or mannose to make sugar acids would be a great way for us to proliferate our product line.’
The acid test
Mascal, currently spending a year as a Fulbright scholar at Chalmers University in Göteborg, Sweden, is also aiming at the speciality market. His team has developed a process that uses hydrochloric acid and gentle heat to digest raw biomass to produce high yields of 5-(chloromethyl) furfural (CMF), a starting point for a number of syntheses.
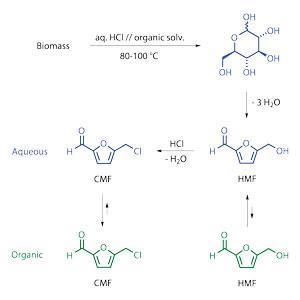
The CMF production process is running at two US pilot facilities with commercial partners. Mascal’s team came across CMF quite by chance during cellulose hydrolysis studies, mistaking it at first for a byproduct. Further investigation suggested that optimising the synthesis could be a ‘game-changer, because CMF is really quite a versatile molecule,’ says Mascal. Key to the process is a two-phase reactor to separate the solvent phase from the aqueous phase, which contains the hydrochloric acid. ‘It allows CMF to be sequestered in the organic phase where it’s safe from the decomposition pathways that have long plagued the acid processing of biomass,’ says Mascal. Yields are over 80% in around one hour and there is ‘no waste stream as such’, because the acid and solvent are both recycled, he adds.
‘If you want to see a great deal of adoption of the technology you have definitely got to come up with some ways that it would give you a market advantage,’ says Mascal, who is now finding ways to use and market CMF. So he is looking for two- to three-step processes from CMF to get to something ‘a little more exotic and interesting’. He has published routes to pharmaceuticals, such as ranitidine (Zantac), and agrichemicals, including a non-toxic herbicide, delta-aminolevulinic acid, which is also used in photodynamic cancer therapy.
Meanwhile, it is ‘very easy’ to make two compounds with a growing market, according to Mascal: 5-(hydroxymethyl)furfural and furan-2,5-dicarboxylic acid. The latter is used to make poly(furan-2,5-dicarboxylic acid) known as PEF, a furan version of PET (polyethylene terephthalate), which can be used to make plastic bottles.
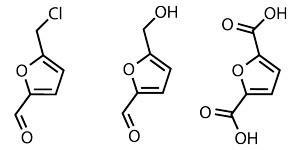
Soft drinks are partly responsible for PEF’s expanding market. In 2011, Coca-Cola announced multi-million dollar partnership agreements with three biotechnology companies, Virent, Gevo and Avantium, to ‘accelerate development’ of the commercial solutions for bottles made solely from plant-based materials. While Gevo and Virent focus on PET made from bio-based paraxylene, Dutch company Avantium uses plant-based materials as feedstocks to produce PEF; it also has a bottle deal with French food company Danone.
Such chemical-based processes have clear advantages over the alternative of using selected microorganisms to digest biomass to produce chemicals, say supporters. ‘The clear advantage is time, because no matter how clever your bugs get they still need time to process whatever it is they are working on – usually days,’ says Mascal. ‘Also, microorganisms don’t work for free. If you have a glucose molecule, two of those six carbons end up as carbon dioxide. A chemical process potentially has a lot of advantages, including the possibility to keep all of the carbon.’
Lovin’ lignin
Mascal’s process to convert raw biomass to CMF leaves behind lignin, a complex cross-linked macromolecule weighing around 10kDa. ‘Lignin is useful stuff,’ says Mascal. ‘We save it [as a powder]. In a commercial facility you could sell the lignin on or you could burn it.’
‘If anybody were ever to come up with a really simple economic way of getting some very useful molecules – simple aromatic hydrocarbons – in a really good yield from lignin, that would be worth a Nobel prize,’ says Mascal. ‘It’s an enormous challenge because it’s such complicated stuff and so highly oxidised. Oxygens are attached directly to the benzene ring. That’s a very powerful bond which is difficult to break.’

‘There is a lot of interest in what can be done with lignin because the cellulosic ethanol plants generate a lot of it,’ says Art Ragauskas from the Georgia Institute of Technology, in Atlanta, US. ‘We have always argued that, since lignin is 20–30% of biomass, then you cannot afford just to say we’ll leave it there and burn it for energy.’
Ragauskas is working on ways to overcome the significant problem of biomass ‘recalcitrance’, the way that lignocellulose’s structural complexity makes it quite resistant to enzymatic hydrolysis used to unlock the cellulose. ‘Recalcitrance is probably the largest sole contributor to the capital and operating costs of converting lignocellulosics to biofuels,’ he says.
‘Five to six years ago, when people talked about recalcitrance, some were sceptical that it would be addressable,’ he says. Now the situation is improving. Ragauskas works with biologists from the BioEnergy Science Center looking at transgenic plants that can reduce recalcitrance while also carrying out in-depth structural studies to gain an insight into the effects of different types of biomass pre-treatment.
Ragauskas is also working on cellulose nanowhiskers, rod-like nanoparticles derived from biomass, which can be added to a wide range of products, from gels to composites, to improve their physical properties. ‘Now people are looking at technologies to orientate the direction and rotation of the whiskers and commercialisation is moving fast,’ he says.
It’s ionic
Imperial’s Welton is excited about his group’s work to extract lignin from biomass using ionic liquids. The discovery was made by chance by PhD student Agniezka Brandt, who was working on dissolving biomass using ionic liquids. When some of the reactions did not work as well as others, Welton asked Brandt to find out why. ‘I’ve selectively taken the lignin out,’ revealed Brandt after further investigation. ‘We were lucky but we had prepared minds. This really does look promising as an approach,’ says Walton.
‘The lignin is partially depolymerised but it is literally in solution in the ionic liquid and that’s what’s so lovely about it,’ he says. ‘For me as a chemist, getting lignin into solution is a huge step forward because it’s so much easier to do reactions on something in solution than it is on solids.’
Welton’s team is working on making the lignin extraction as efficient and effective as possible. They now know that the ionic liquid’s anion does the biomass bond-breaking and they are trying out different cations in the ionic liquid to tweak its physical properties and optimise the process. For example, changing the viscosity of the ionic liquid could help it to soak into the wood.
Yet some have their doubts about using ionic liquids on an industrial scale. ‘They have fantastic properties for very small scale applications where you are making high-value materials, but for high volume work, ionic liquids are probably not going to be economically feasible to use,’ says Mascal.
Post-petroleum polymers
Meanwhile, Welton has a new project to investigate ionic liquid biorefining of lignocellulose to sustainable polymers, sponsored by the UK’s Engineering and Physical Sciences Research Council. He outlines three ‘synthetic challenges’ for renewables. The first, he says, is to get a new source of a compound in use today. Second is to make materials with properties that match those of chemicals currently in use and third is to create large volumes of chemicals that have new properties and then discover what the chemicals could be used for.
Welton is particularly taken with the second challenge, which his polymer project targets. ‘It’s not the sophisticated chemicals that will be a problem [in a post-petroleum era]. We will find a way of making all the sophisticated chemicals that we want, as we do now,’ he says. ‘We really need to think about the low-cost, high-volume, high-mass materials.’ He looks at all the plastic items on his desk, from the folder that holds his lecture notes to his pen holder. ‘What are we going to make those kind of things from, the really mundane stuff that we take for granted that is all around us?’ he asks.
‘It has to be very simple, based on what’s available and the sophistication of the synthesis has to be really, really good because the margins will be so tiny. So that puts an amazing discipline on the chemistry that you’re going to have to invent,’ says Welton. ‘For me, as a chemist, I find that interesting.’
Emma Davies is a science writer based in Bishop’s Stortford, UK
Biomass bugs
Microorganisms have always played a key role in renewable chemicals and fuel production. US biotech company Verdezyne produces renewable chemicals from plant oils using engineered yeast strains. The process appears to work on any oil but the ‘feedstock of choice’ is free fatty acid side-streams from vegetable oil processing, says chief executive William Radany.

The company’s first products are a set of acids used in nylon production and traditionally produced from petroleum products: adipic, sebacic and dodecanedioic acids with chains of six, 10 and 12 carbon atoms respectively. ‘Unengineered organisms would chew up the fatty acids two carbons at a time until they had eventually used up all of the hydrocarbon,’ says Radany. Verdezyne has used genetic engineering to alter metabolic pathways and enzyme processes so that the organisms stop chewing at the required number of carbons. The company also has a programme for production of methyl methacrylate, used to make polymethyl methacrylate acrylic plastics.
The plan is to go commercial with dodecanedioic acid by the first quarter of 2014, says Radany. ‘We anticipate renting out somebody’s processing and fermentation equipment to do that. We feel that our process has sufficient economic advantage that we will be able to do that and be profitable.’
Meanwhile, LanzaTech, founded in New Zealand in 2005 and now with headquarters in Chicago, US, uses native gas-eating organisms to turn industrial carbon monoxide emissions into fuel and chemicals: ethanol and 2,3-butanediol (BDO) in the first instance. The company has a pilot project to convert waste gases from a New Zealand steel mill and is currently commissioning a second pre-commercial facility in China using steel mill off-gases for ethanol production. The plan is to commercialise BDO by the end of 2014.
The company also has an organism that converts carbon dioxide in the presence of hydrogen into acetic acid. Petronas, Malaysia’s national oil company, is investing in the technology and construction of a demonstration facility is scheduled for the second half of 2013. The carbon dioxide will come from industrial waste gas and the hydrogen from a chemical plant.












No comments yet