It is 80 years since James Chadwick discovered the neutral sub-atomic particle and 40 years since the Laue-Langevin Institute opened its doors. To celebrate, Philip Robinson visits the most intense neutron source in the world
It is 80 years since James Chadwick discovered the neutral sub-atomic particle and 40 years since the Laue-Langevin Institute opened its doors. To celebrate, Philip Robinson visits the most intense neutron source in the world

Just as today’s physicists across the Alps at Cern are turning subatomic particles inside out to uncover the source of the universe’s missing mass, their forebears were perplexed by a similar conundrum. The mismatch between the mass of an atom and its atomic number was only solved by James Chadwick’s discovery of the neutron 80 years ago. Since then, the neutron has become one of science’s most powerful allies, with researchers from around the globe flocking to sites such as the ILL to make use of neutrons in their research.
Neutrons are a unique and versatile probe of atomic structure and dynamics. When they are fired at a material at just the right speed, they have a wavelength that is on the same order as interatomic distances. This means that they are diffracted by the material’s atoms, producing diffraction patterns that reveal the nature of a sample’s structure, in a similar way to x-rays. When it comes to diffraction experiments, ‘99% of the time, you use x-rays’, says Andrew Harrison, the ILL’s director, acknowledging that x-ray sources are considerably cheaper than nuclear reactors. But he adds that neutrons have very distinct properties that make them indispensable analytical tools.
The attraction
In contrast to x-rays, neutrons interact with the nuclei of atoms, rather than the electron cloud, allowing atom positions to be determined with higher accuracy. This interaction between x-rays and electrons means their ability to ‘perceive’ an atom is proportional to its number of electrons, all but disappearing for hydrogen. But an atom’s ability to scatter neutrons varies almost randomly from element to element, and over a much smaller range, so the technique is sensitive to hydrogen, carbon and oxygen, even in the presence of heavier elements.
This variation even extends to isotopes of the same element: hydrogen and deuterium scatter neutrons so differently that samples can be deuterated to highlight specific areas or species of interest. And because the neutron has no charge, it can travel deep into a material, unaffected by the electromagnetic interactions that make such journeys impossible for x-rays.
There is perhaps some poetic irony that a neutral particle should have such an incredible attraction, drawing not only researchers but also other world-leading facilities. Since the ILL produced its first neutron 40 years ago, the European synchrotron radiation facility and an outstation of the European molecular biology laboratory have both become neighbours to the ILL. ‘There are tens of thousands of scientists and engineers on site – arguably the highest density in Europe,’ Harrison told Chemistry World during a recent visit to the facility.

At the centre of the site is the ILL’s nuclear fission reactor, where atoms of uranium are split to release neutrons. Standing in the adjoining reactor hall, it is hard not to regard the engineering feats required to harvest, transmit and detect these tiny particles with some awe. One such feat is the neutron guide, the man-sized tubes that carry neutron beams away from the core and towards different experimental stations. Here, they might interact with cell membranes, superconductors or the blades of a jet engine before their story ends as they combine with 3He atoms in a detector.
‘Probably the biggest story we’ve had this year is that of the magnetic soap,’ says Harrison. This project, led by Julian Eastoe at the University of Bristol, UK, created a liquid surfactant that, through the incorporation of iron ions, was imbued with magnetic properties (Chemistry World, March 2012, p30). Such a magnetic soap could be a simple means of clearing up oil spills, for example, or for straightforward extraction of components from a mixture. But the source of the magnetism was a puzzle. ‘They knew they had a magnetic effect,’ says Isabelle Grillo, a member of staff at the ILL who participated in the experiment, ‘but they didn’t know why – if you just dissolve iron in water, it doesn’t have any magnetic properties.’
Solving this puzzle demanded the structural understanding that only neutrons can provide. In particular, the neutron’s differing sensitivity to hydrogen and deuterium allowed the micellar structure to be investigated. ‘By replacing hydrogen with deuterium, we can tune the contrast of the sample [relative to the solvent],’ says Grillo. In the case of a micelle, this tuning can be used to make different parts of the structure stand out against the solvent background, so the whole micelle or just the core or the surfactant layer can be highlighted. Examining the core revealed that within the micelles the iron ions were closely arranged – close enough to interact and produce magnetism.
Modelling membrane mechanics
Lending her expertise to groups from around the world, Grillo is typical of the staff responsible for the ILL’s instruments: in equal parts technician, consultant, analyst and researcher. For Robert Barker, one of the ILL’s instrument scientists, evangelist is an important addition to the list. ‘The interesting stuff comes with the external groups,’ Barker says. ‘So I go out to conferences and talk to people to show them what we can do.’ And what Barker can do is of particular interest to biologists – investigating biophysical phenomena in proteins and cell membranes, which bristle with the hydrogen atoms that neutrons so expertly recognise.
Barker specialises in artificial membranes. ‘If we put a whole cell in the neutron beam, there’s just too much going on, too much information,’ he says. ‘So we simplify it, using a model to focus on a particular thing.’ These models allow biologists to investigate membrane mechanics on the angstrom scale in a way that is biologically relevant. ‘A real membrane has fluctuations, it moves,’ he explains. ‘But an artificial membrane deposited on a surface doesn’t. We’ve grafted a lipid layer on to a surface and deposited a membrane on top so it is completely separated from the surface.’
Barker’s findings may also have implications for patient safety. ‘Where the ethanol inserts is also where you often see cholesterol in the membrane,’ he explains. ‘So if you have increased cholesterol levels, that may affect the closure mechanism and this may be something that should be taken into account [in calculating anaesthetic doses].’
Another ILL staff member with an interest in biology is Mark Johnson, who works in the ILL’s computing for science group. He uses neutrons to measure the elasticity of DNA, exploiting yet another capability of neutron diffraction – inelastic scattering. In these experiments, the energy a neutron gains or loses as it interacts with the atoms of a sample reveals the dynamic properties of the sample. In Johnson’s case, this involves measuring the vibrational modes, or phonons, within crystalline DNA.
‘The elastic properties of DNA allow it to stretch and bend, to interact with proteins for example,’ says Johnson. ‘And one of the fundamental quantities in such interactions is the stiffness.’ Although this property has been measured previously using x-ray scattering, Johnson explains that the measurement was made on the length scale of a single DNA base pair distance – just 3.3Å. ‘The helix turn is 33Å, so they weren’t measuring the effect of the helix at all,’ he reveals. ‘We needed better resolution than you can get with inelastic x-ray and we can now do this because we rebuilt one of our instruments, so it has fantastic resolution that allows us to go to much longer length scales.’
At this improved resolution Johnson’s measurements gave a value significantly higher than anything previously reported and he is confident that this is the ultimate value for the intrinsic stiffness of DNA. ‘We’ve made the only measurement on the length scale of the helix and this can now feed into the accurate parameterisation of DNA interactions,’ he says.
Looking back in time
Scrutinising a different kind of cell and membrane altogether, Thomas Hansen, an ILL crystallographer, applies his expertise to the study of fuel cells, and specifically the oxygen-conducting membranes they require. ‘If you want to know which way oxygen moves in a solid material, and what you need to do to make it move more, the neutron sees that precisely,’ Hansen explains. He adds that the materials usually employed for these membranes make them unsuitable for x-ray investigations. ‘The most famous is example yttrium-doped zirconia,’ he explains. ‘Yttrium and zirconium are relatively heavy atoms, which makes it very difficult to see the lighter oxygen in there [with x-rays].’

‘You have many little crystals making up the axe,’ explains Hansen. ‘If hammering and rolling was used [to make it], the crystals have some preferred alignment, and the neutron diffraction response will depend upon the sample’s orientation in the beam. But a perfectly random distribution will give the same response in any orientation.’ X-ray diffraction can also probe these structural features, but the neutron’s ability to penetrate deep into the material made it the only choice in this instance. ‘With x-rays, you will only see the surface,’ says Hansen. ‘With neutrons you see the whole axe – and without having to break up the precious sample.’
Although knowledge of forging techniques existed in Ötzi’s time, the results showed that the axe had not received any strengthening treatment. This led to the interpretation that the axe was a decorative status symbol, rather than a tool or weapon, indicating something about Ötzi’s social standing. But Hannsen is careful to confine the ILL’s contribution to the neutron evidence. ‘Here we must draw a line: it’s not up to us to do the final interpretation – that is the job of the archaeologist. And so it is with other fields.’
Worth the candle
Hansen’s colleague, Andrew Wildes, likens the ILL to a recording studio, and its staff to record producers with ‘a bit of session musician in there as well – you get to play on the record’. But he also notes that ‘recording time’ in a nuclear reactor does not come cheap and, for all their uses, neutrons are not without limitations. ‘The problem with neutrons is that there’s just not that many of them,’ he explains. ‘The number of neutrons produced here, at the most intense source in the world, is about the same as the number of photons produced by a candle.’
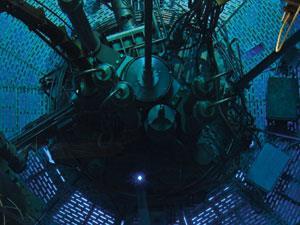
And it isn’t just materials that are under investigation at the ILL, as the neutron itself is still a significant area of study. Even 80 years after its discovery, this particle still holds a few secrets scientists want to uncover, such as whether or not it has an electric dipole. ‘Although the particle is neutral, there are theories that predict it has a charge separation within it,’ says Harrison. But the magnitude of that separation is equivalent to placing a proton and an electron at opposite ends of the earth, so measuring it requires incredible precision. ‘It’s one of the most elegant but difficult experiments – it’s been going on for 40 years without a result,’ Harrison explains. But every year brings the answer closer: ‘When experiment started, they were about a million times out in terms of precision. Now they are about 50 times out.’
The future of neutron science in Europe lies with the proposal to build the European Spallation Source (ESS), a facility in Lund, Sweden. The ESS should succeed the ILL to become the world’s brightest source of neutrons but the time to hand over is still some way off, Harrison notes. ‘If the ESS get the funding tomorrow, it’ll be 2030 before these guys are up to strength, so we have another 15 years of maintaining our position as the world leader.’ And in that time Harrison intends to ensure that neutron science continues to get the recognition it deserves. ‘I want [UK science minister] David Willetts to read about neutrons while he’s eating his Rice Krispies,’ he smiles.













No comments yet