Robert Robinson pioneered the use of curly arrows to show electron movement. David O'Hagan and Douglas Lloyd report on this eminent historical figure

Sir Robert Robinson (1886-1975) was one of the giants of early 20th century organic chemistry. He played a seminal role in our understanding of chemical reactivity and made outstanding and extensive contributions to the synthesis and structure determination of natural products, particularly the alkaloids. In his later career he held the most senior positions in UK science as president in turn of the Chemical Society, the Society of Chemical Industry and of the Royal Society. His eminent work also earned him international recognition in the form of the 1947 Nobel prize in chemistry.
Born in Rufford, Derbyshire, on 13 September 1886, Robinson was the son of a surgical dressing manufacturer. He carried out his undergraduate and PhD research at the University of Manchester, the latter under the supervision of William Perkin Jr. He then followed an academic career path, holding a number of short professorial residencies prior to moving to the University of Oxford in 1932. He remained at Oxford until his retirement in 1955.
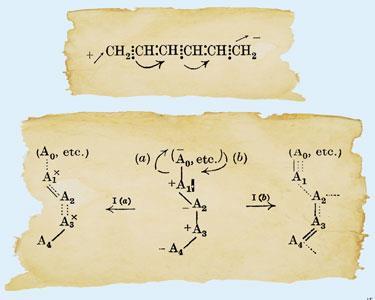
The University of St Andrews was one of the stepping stones en route to Oxford, and Robinson was based there from 1921 to 1923. During this two year period he published 24 papers, two of which remain widely cited today. The papers presented fundamental contributions to developing ideas on ‘electronic theory’. One, published with James Wilson Armit (a local baker’s son), had the first illustration of an aromatic ring with a circle in the centre of a hexagon to represent delocalisation; a paper which has been widely acknowledged in discussions on the history of benzene and aromaticity. The second paper published in 1922 – and the focus of this feature article – contained the first illustration of the curly arrow as a device to represent electron movement in conjugated organic molecules. Robinson coauthored this second paper with William Ogilvy Kermack.
William Ogilvy Kermack
Kermack was a Scotsman born in Kirriemuir in 1898. He studied maths, natural philosophy and chemistry at the University of Aberdeen (graduating in 1918) and then moved to the Dyson Perrins lab at the University of Oxford for two years with William Perkin Jr to work on the alkaloid harmaline. He returned to Scotland to the chemicals section of the Royal College of Physicians in Edinburgh where he remained until 1949.

In 1922 Kermack collaborated with Robinson but it appears that they never actually worked in the same laboratory, as one was in Edinburgh and the other in St Andrews.
At age 26, two years after the curly arrow paper, Kermack was tragically blinded when caustic alkali exploded in his eyes. Showing considerable fortitude he was married one year later and carried on as a research chemist in alkaloid and carbohydrate chemistry for many years.
His interests extended to the statistical analysis of fertility and death rates in urban and country populations and he made seminal and enduring contributions to the emerging science of epidemiology where he and his colleague Anderson McKendrick modelled epidemic episodes of infectious diseases now known as the Kermack–McKendrick theory.
He was appointed the first professor of biological chemistry at the University of Aberdeen in 1949. Despite his total blindness he rose through the academic ranks, with papers being read daily by his secretary and colleagues. He became dean of science in 1961. He remained at Aberdeen until his formal retirement in 1968, and died working at his desk in 1970.
Gaining mechanistic insight
Together these two papers initiated a major transition in organic chemistry, moving the international focus from structure elucidation to understanding reaction mechanism. Like many transitions in science there was much to evaluate and fierce rivalries ensued in establishing the ground rules in what would subsequently be called ‘physical organic chemistry’.
In the 1922 paper, Robinson used the curly arrow to describe the reactivity of the molecule 1,3,5-hexatriene. His interest in hexatriene lay in the fact it is closely related to butadiene – a molecule that was somewhat of a hot topic at the time. It was well recognised that if butadiene is treated with bromine, a mixture of 1,2- and 1,4-dibromobutenes formed. But an understanding of why was yet to be developed.
In 1899, the German chemist Johannes Thiele had put forward a ‘theory of partial valencies’ to describe double bonds. He proposed that the double bond in ethene consisted of a full covalent bond with a second partial bond, allowing each carbon atom to retain a partial valency pointing into space. The partial valencies were believed to be predisposed to react with bromine, for example, to make 1,2-dibromoethane. By 1922, Thiele’s ideas had been extended to try and understand the structure and reactivity of benzene, forming a useful working hypothesis given the information available at the time. Rationalising this for butadiene, however, was less satisfactory as the two dibromo isomers were generated.
In the 1922 paper, Robinson offered a more satisfactory hypothesis much closer to a modern understanding of conjugation. In developing his ideas, he drew on the theories of the US chemists Gilbert Lewis and Irving Langmuir - concerning two electron covalent bonds and the stability of octets of electrons - and those of UK chemist Arthur Lapworth on alternating polarities.
Robinson and Lapworth
Lapworth was a close colleague and confidant of Robinson. Their relationship began in 1909 when Lapworth arrived as a teacher at the University of Manchester, where Robinson was carrying out graduate research. Robinson stated that they had, ‘unlimited opportunity for discussion from that time until 1912’. Talking about his friend’s research at that time, Robinson said: ‘[he] was obsessed by his theory of alternate polarities, though not a reliable guide in all circumstances, [it] still provides a useful mnemonic for the behaviour of molecules of many carbon compounds in reactions.’

In 1912, Robinson moved away from Manchester to take a chair at the University of Sydney in Australia. He returned to the University of Liverpool in 1915, and then, after a short period with the British Dye Stuffs Corporation, arrived at St Andrews in 1921. This was a busy eight years, during which time Robinson and Lapworth had both continued to develop their ideas on electronic theory.
Lapworth had deeper roots in the subject. As early as 1898, he alluded to finding a unifying concept of reactivity to ‘demonstrate that it is possible to refer the majority of reactions and changes in organic chemistry to necessary variations of at most one or two simple laws’. In 1904, he had recognised that cyanide attacks a ketone to generate a cyanohydrin, and had proven this by the isolation of crystalline salts of cyanohydrins. He had also demonstrated that the process was acid catalysed and reversible. He rationalised that this reactivity was due to the polarity of the carbonyl and the anionic nature of cyanide, leading to his recognition that oxygen polarised the C=O bond and rendered the carbon electropositive. It followed that the next carbon would be negative and the next hydrogen positive ie induced alternate polarities. Robinson stated in Lapworth’s Royal Society obituary: ‘when in the early Manchester days, one discussed synthetical projects with Lapworth, it was quite clear that he had some unusual private way of deciding whether they would "go" or not. It turned out to be a scheme of "alternating polarities" in a chain of atoms and the theory was published in 1920.’
Despite their long friendship, in 1922 Robinson and Lapworth published separately, but simultaneously. In each case this independence was tempered with generous acknowledgement of each other in their respective papers. However they did not read each others manuscripts in advance, indicating that they were working in a fast moving competitive field.
All in a muddle
In Lapworth’s paper, curly arrows also appear; however they represent the movement of partial valencies. It is difficult now to appreciate this model, as it doesn’t follow modern convention. A covalent bond was assumed to have three ‘partial valencies’ and his arrows move two of these, thus the connection that an arrow moves two electrons is obscured, although Lapworth was very close to describing the modern convention. Lapworth’s focus was on aliphatic carbonyl chemistry, and he was developing a model to encompass cyanohydrin formation as well as enolate formation.
The Robinson and Kermack paper, on the other hand, focused on conjugated and aromatic systems, and was the easier to assimilate. That’s not to say it was immediately understood, and these new ideas proved difficult for the chemical community to grasp. An unseemly scramble then ensued to present a more clearly accessible rationale.
Others such as Bernard Flürscheim, who had studied under Thiele, and the fast emerging Christopher Ingold at Imperial College London also became involved. In the few years following the 1922 papers, the controversy was so intense and so confusing that the Chemical Society refused to receive any more papers on the subject from these leading participants.
A 1925 private correspondence from Martin Lowry, a professor of physical chemistry at the University of Cambridge, to Robinson illustrates the extent of the community’s confusion: ‘I have not yet got used to your new system of arrows and want a little practice in order to think in terms of them. Even now I am not quite sure whether they represent a transfer of one electron or two. My rather vague impression is that in the earlier papers you generally transferred only one, while in your last C and I [Chemistry and Industry] paper you appear to transfer two. It may not matter much which scheme is used, but I want to get a clear picture in my own mind of your present view of this mechanism. I hope you don’t mind being bothered like this with elementary questions; but in view of the fact that so many people misunderstand, more or less willingly, the Manchester doctrine, it seems worth while to make sure that there are at least a few people about who willingly understand it.’
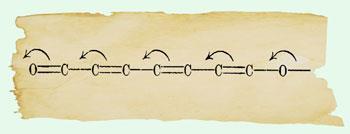
Indeed, in the Robinson and Kermack 1922 illustration it appears that only a one electron transfer is represented by the curly arrow. The electron count is clearly illustrated in the before and after structures of butadiene, and by implication extends to the hexatriene structure. However in 1924 and 1925, Robinson used the curly arrow notation widely, and confidently illustrated two electron movement in a manner akin to contemporary usage (below).
Articulate Ingold
Ingold was particularly energetic in developing an understanding of aromatic reactivity, and was the better articulator of the concepts of the new electronic theory. Subsequent years would see Robinson and Ingold go head to head for priority, both on theory and development of terminology. Ingold’s notation gained widespread acceptance, with his nucleophile and electrophile terminology successfully displacing Lapworth’s anionoid and kationoid (the terms used by Robinson). The pair also presented contradictory conventions for the positive and negative signs attached to electron donating and withdrawing groups, with Ingold’s winning out.

Robinson was rather bitter in that Ingold took the foreground and states in his memoir of 1976: ‘I am touching on this question of acknowledgement as [Ingold] was apt to include a necessary reference to Lapworth or myself in a large number of references so that any idea that our contributions are original or applicable to the matter in hand was well and truly buried.’
By contrast it is interesting to note that Lapworth’s last published output in 1931 was a collaboration with Ingold on directing groups and reactivity in aromatic nitration, and clearly they had reconciled any differences they may have had. Lapworth was restrained and gentle by character and as Robinson states: ‘there must have been occasions when Lapworth was angry, but I cannot recall one, and I am quite sure he never did a mean action.’
The curly arrow was of minor significance in the 1922 St Andrews paper, however its simplicity and power as a symbol of electron movement resulted in its widespread and rapid uptake, to the point now where it holds an iconic status in organic chemistry.
David O’Hagan is head of organic chemistry, and Douglas Lloyd is professor emeritus, at the University of St Andrews, UK
Further reading
W O Kermack and R Robinson, J. Chem. Soc. Trans., 1922, 121, 427
A Lapworth, J. Chem. Soc. Trans., 1922, 121, 416
R Robinson, Memoirs of a minor prophet, Elsevier, Amsterdam, the Netherlands, 1976
T I Williams, Robert Robinson: chemist extra-ordinary, Oxford University Press, US, 1990
Profile of Christopher Ingold: Chemistry World, December 2008, p50
Profile of the Perkin family: Chemistry World, March 2010, p54










No comments yet