Living factories provide alternative to complicated chemical syntheses or farming hectares of crops
Chinese scientists have taken a biosynthetic pathway from plants and introduced it into bacteria to create potentially health-boosting chemicals. Their route provides an alternative to complicated chemical syntheses or farming hectares of crops.
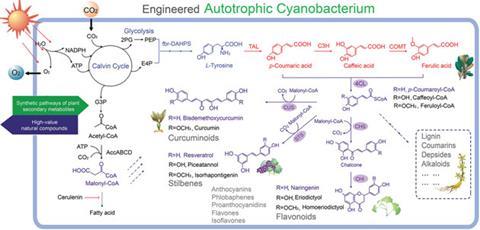
Shared photosynthetic components between plant chloroplasts and cyanobacteria make these microbes ideal hosts for expressing foreign plant enzymes. Ping Xu and colleagues at the Shanghai Jiao Tong University have genetically engineered the cyanobacterium Synechococcus elongatus PC7942 with plant-derived enzymes. In total, the team created 18 bacterial strains expressing different combinations of enzymes. The different strains generate a variety of compounds with a six-carbon, phenyl group and three-carbon propene tail, called phenylpropanoids.
Phenylpropanoids perform diverse functions in plants, ranging from ultraviolet light protection to pathogen defence. One such compound, resveratrol, is made when the bacteria express the plant enzyme stilbene synthase downstream of enzymes tyrosine ammonia lyase and 4-coumarate:coenzyme A-ligase. Found in the skin of grapes and other berries, resveratrol reduces the risk of heart disease and is a valuable pharmaceutical commodity. Different versions of the engineered bacteria can also churn out the phenylpropanoid antioxidants caffeic acid, naringenin and coumaric acid.
What’s more, the team added feedback-inhibition resistant enzymes to the bacteria so that the chemical yields would surpass physiological levels. Photosynthesis within the cyanobacteria generates the chemicals from just water, carbon dioxide and a few mineral nutrients.
The bacterial growth medium houses the products, but isolating them at an industrially relevant yield is currently the biggest challenge. However, by not needing to harvest crops, generating the compounds from bacteria is potentially more sustainable. Xu stresses the potential of this point: ‘For the production of 1 tonne of natural resveratrol, our method may save about 485 hectare of farmland at its current production level.’
‘The approach deftly sidesteps major economic challenges by targeting chemicals with high intrinsic value,’ comments Paul Fowler, executive director of the Wisconsin Institute for Sustainable Technology in the US. A world-scale production plant under these circumstances is not a pre-requisite for commercialising this research.’
References
This article is free until 06 June 2016
J Ni et al, Green Chem., 2016, DOI: 10.1039/c6gc00317f









No comments yet