Warnings that completion of final steps in opiate biosynthesis could be a double-edged sword
Science policy experts have called for urgent measures to be put in place to prevent strains of yeast that are capable of producing opiate drugs from falling into the hands of criminals. The prospect of ‘home-brewed heroin’ has been raised after new research describes how a key enzyme in the pathway from glucose to morphine and other opiates has for the first time been successfully expressed in yeast. The finding means that the complete biosynthetic pathway for the family of compounds that includes codeine and morphine is close to being achieved in yeast. However, opinion on the potential dangers to society of the new technology is divided, with other experts suggesting that there are unlikely to be major problems.
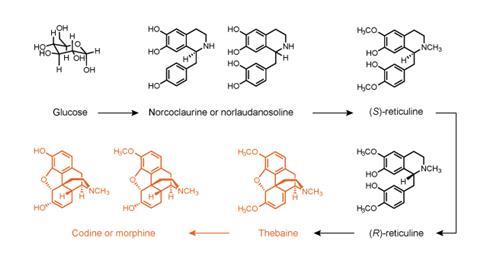
For several years a number of research teams have been inserting plant genes into microorganisms to reconstruct the pathways that produce a class of compounds called benzylisoquinoline alkaloids (BIA), a family of around 2500 compounds, many of which have pharmacological activity – including codeine and morphine. If it were possible to produce these compounds cost-effectively through fermentation, it would provide a more secure supply of important drugs and would allow scientists to investigate – and potentially create – new compounds within the BIA family.
However, the pathway is complex, with some 16 steps, and incorporating all the stages within a single organism has proved challenging. Several of the ‘downstream’ stages of the pathway have been described in yeast, but further upstream it has not yet been possible to find the relevant enzymes that are happy to function in the yeast cell.
Chemistry beet
One key sticking point has been to identify an enzyme, a tyrosine hydroxylase, that would work in yeast to selectively convert tyrosine to L-DOPA, a key step along the pathway. Now, a team led by John Dueber of the University of California, Berkeley, US, has succeeded in identifying such an enzyme. To do this, the researchers engineered yeast cells to express an enzyme capable of converting L-DOPA into a fluorescent pigment, betaxanthin. This is in effect a biosensor, detecting whether the desired product, L-DOPA, has been produced. They then intended to screen multiple tyrosine hydroxylase candidates, but happened to find one almost immediately, derived from the beet plant.1
‘With our biosensor system, fluorescence indicates whether L-DOPA has been produced in the cell,’ says Dueber. Furthermore, the intensity of the fluorescence corresponds to the concentration of the L-DOPA. This allowed the research team to mutate the enzyme to achieve higher levels of activity.
The finding enabled the team to reconstruct the early part of the BIA pathway in yeast. This now opens the way to stitch together all stretches of the biosynthesis from glucose to useful end products, something that Dueber thinks may be possible within the next two or three years.
‘We realised this field was moving much faster than we had anticipated and that while there are many potential benefits, there is also the possibility for the illicit use of the technology. We need to anticipate rather than react to any potential problems.’
Brewing bad
For this reason Dueber contacted technology policy experts Kenneth Oye and Chappell Lawson of the Massachusetts Institute of Technology, US, and Tania Bubela of the University of Alberta in Canada, to garner their views. In a commentary paper,2 Oye, Lawson and Bubela say: ‘In principle, anyone with access to the yeast strain and basic skills in fermentation would be able to grow morphine producing yeast using a home-brew kit for beer-making.’
The authors suggest a number of measures to head off this possibility, such as engineering the yeast to require particular nutrient supplements that are difficult to identify. They also suggest increased security of laboratories, and a requirement for gene sequence suppliers to be aware of the key sequences and to have these flagged up if they are ordered.
John Collins, coordinator of an international drug policy project at the LSE in the UK, says: ‘If these yeasts allow illicit drugs to be produced in meaningful quantities in the countries where they are consumed, then it could become a game-changer in terms of control. If it proliferates, I don’t think there would be an easy answer – there is not an answer to the current supply chain.’
Paul Nightingale of the science policy research unit at the University of Sussex, UK, believes that existing legislation is likely to be sufficient to meet any concerns. ‘In the UK certainly such things would be covered by GMO [genetically modified organisms] regulations and the misuse of drugs laws. There are also European regulations relating to non-release of certain organisms. I don’t think this is something that anyone could get easy access to and get it out of a lab.’
A former UK government advisor on drugs policy and former head of the Drugs Intelligence Unit at the Forensic Science Service, Les King, is also sanguine. ‘I don’t see a legal problem here. Pharmaceutical manufacturers will have a licence to grow the yeast and extract the opiates from the culture medium,’ he says. ‘I would imagine it could be a lot more efficient than extracting opiates from opium. I would guess that a particular strain of yeast will produce one type of opiate. I don’t see any opportunities for illegal diversion or home-grown production. Opium poppies grow in my garden – in fact they grow anywhere, but no drug users bother to collect the opium for smoking.’
References
1 W C DeLoache et al, Nat. Chem. Biol., 2015, DOI: 10.1038/nchembio.1816
2 K Oye et al, Nature, 2015, 521, 281









No comments yet